Coupling the temperature dependence of microbial and plant spatial turnover rates based on eco-metabolic theory
Coupling temperature-dependent spatial turnover of microbes and plants using the metabolic theory of ecology
Article,April 2023,Volume238, Issue1,Pages 383-392
New Phytologist, [IF 10.32]
DOI:https://doi.org/10.1111/nph.18695
Original link: https://nph.onlinelibrary.wiley.com/doi/10.1111/nph.18695
First Author: Xiao Xian
Corresponding author: Liang Yuting
Co-authors: Ma Zhiyuan, Zhang Jiabao, Sun Bo, Zhou Zhong
Main units: Nanjing Institute of Soil Science, Chinese Academy of Sciences, Changzhou University, University of Oklahoma, Tsinghua University, Lawrence Berkeley National Laboratory
Summary
There is an urgent need to understand the coupled relationship between belowground microorganisms and aboveground plants in response to temperature under the background of climate change. Metabolic theory of ecology (MTE) provides a way to predict metabolic rates and species diversity, but the spatial scale dependencies and connections between plants and microbes remain unclear. This study answers this question with two independent datasets: one is a rice field sampling dataset targeting soil bacteria and microbial functional genes, and the other is a global dataset including microorganisms (bacteria, fungi and archaea; n = 139 ) and metadata sets of plants ( n =206). The results showed that the spatial turnover rate of bacterial communities and microbial functional genes increased with increasing temperature, which was consistent with the MTE theory. The results of the meta-analysis showed that the temperature-dependent patterns of spatial turnover rates could be further extended to the global scale, and the spatial turnover rates of microorganisms and plants were consistent with the MTE theory. Even controlling for ambient temperature, there was a significant correlation between belowground microbes and aboveground plants, suggesting that factors other than their common relationship with temperature also contribute to their connection. These results demonstrate the broad application of MTE theory in biology and its significance in predicting the ecological consequences of future climate warming.
introduction
Metabolic theory of ecology (MTE) predicts a quantitative relationship between an organism's metabolic rate and ambient temperature, ie higher temperature will increase individual expressive forces such as developmental rate, mortality and lifespan. This dynamic process is expressed by the Arrhenius/Boltzmann exponential relationship, namely R∝e -E/kT , where R is the rate of a given biological process (such as mortality), e is the base of the natural logarithm, and k is the Boltzmann constant ( 8.62×10 -5 eV/K), T is the temperature in Kelvin, and E is the "activation energy" characterizing the dependence of a given biological process on temperature. This framework can be further extended to the population and community levels of ecosystems, since many characteristics of populations and communities depend on the performance of individual organisms, such as species diversity (alpha diversity). For example, Allen et al. showed that the species richness of large organisms such as European trees, North American tiger beetles, and European amphibians increases with increasing ambient temperature according to ecological metabolic theory. From subalpine Colorado to tropical Panama, the relationship between the species diversity of forest soil microorganisms and temperature was also confirmed on the large latitude temperature gradient, but their temperature dependence (E = 0.13~0.47 eV) was lower than that of trees and Recorded values for animals (~0.65 eV). Although MTE applies to the α-diversity of macroorganisms and microbes, it is unclear how the β-diversity of their spatial-scale patterns changes under large temperature gradients and whether MTE also applies to these patterns.
Biological metabolism influences the flow of energy and matter in an ecosystem, therefore, the MTE theory can be extended to ecosystem processes such as biomass production. Yvon-Durocher and Allen found a temperature dependence ( E = 0.27) of short-term total primary production (i.e., total photosynthetic flux) based on aquatic ecosystem experimental data . In addition, the biomass accumulation rate after forest disturbance was also temperature-dependent, and the biomass accumulation rate was a function of the inverse of temperature (1/ kT ) ( E = 0.32). However, few studies have assessed whether microbial biomass production is consistent with MTE, although many cases have demonstrated the sensitivity of microbial biomass carbon to changes in ambient temperature. For example, four different soils were incubated for 14 d along a temperature gradient of 5–45°C, and a significant increase in soil microbial biomass carbon was observed before the breakpoint temperature and decreased after the breakpoint temperature. Curtin et al. found that soil microbial biomass carbon was greatly reduced (18-35%) under a temperature gradient of 5-25 °C in a laboratory culture lasting 85 days, which significantly enhanced the temperature response of carbon mineralization. Therefore, given the critical role of microbes in climate feedbacks, empirical evidence is needed to reveal the potential link between ambient temperature and soil microbial biomass over large temperature gradients.
In this study, the author proposes three hypotheses (Figure 1):
1. It is assumed that the spatial turnover rate of species (β-diversity) conforms to MTE, and this theory is applicable to subterranean microbes (i.e., soil major microbiome: bacterial communities and microbial functional genes) and aboveground macrophytes on a global scale. This is because the spatial turnover rate of species reflects the community level of ecological organization, which should be affected by temperature.
2. As indicators representing ecosystem processes, it is assumed that belowground microbial biomass and aboveground plant biomass also fit the MTE, consistent with widely studied pelagic ecosystems. At the same time, the authors speculate that there are interdependent relationships between the spatial scales or functions of aboveground and belowground ecosystems.
3. Assume that community-level traits (i.e., spatial turnover) are related to ecosystem processes (i.e., biomass production) because the two are inextricably linked.
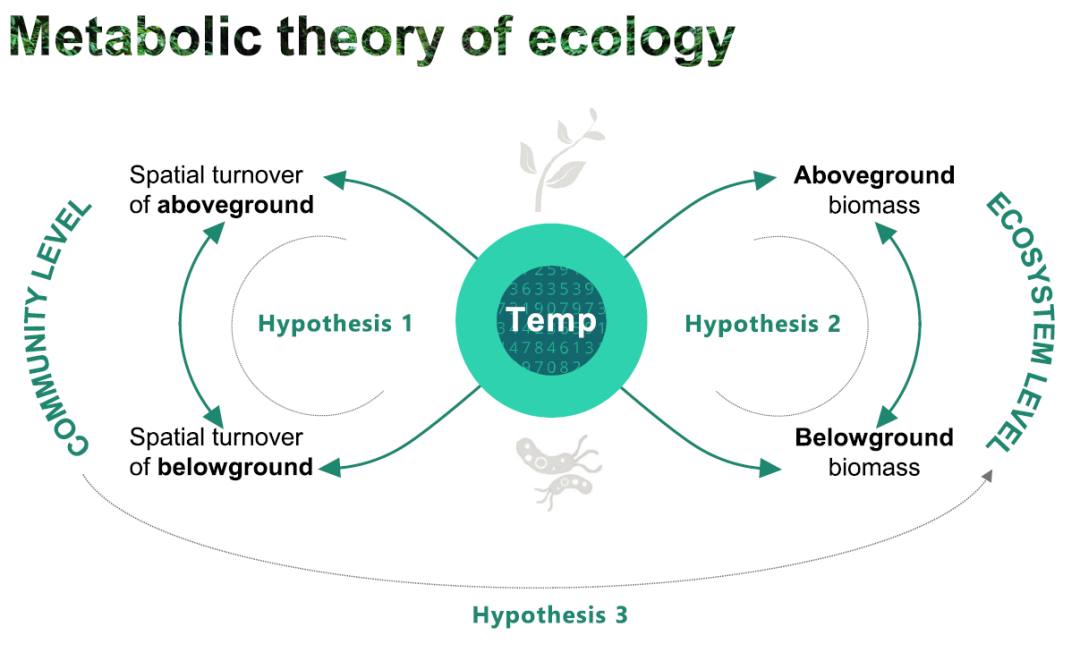
Figure 1. Hypothetical schematic diagram
Hypothesis 1: The spatial turnover rate of species conforms to MTE, which applies to belowground microorganisms and aboveground macrophytes. Hypothesis 2: Belowground microbial biomass and aboveground plant biomass reflect ecosystem processes and are also consistent with MTE. The authors expect aboveground and belowground ecosystems to be interdependent on spatial scale or function. Hypothesis 3: Community-level traits are related to ecosystem processes because the two are inextricably linked.
To test these hypotheses, the authors selected 39 paddy fields in 13 regions in China's major rice-growing regions (19.75°N ~ 47.58°N, 110.41°E ~ 126.92°E) to study the spatial turnover of soil bacterial communities and microbial functional genes rate, as well as total belowground microbial biomass and aboveground rice biomass. Paddy soil ecosystem was chosen because compared with dryland soil, the heterogeneity of the soil environment (such as soil moisture, oxygen content, and organic composition) in paddy fields is small, which reduces the impact of soil heterogeneity on ecological processes to a certain extent, so Paddy fields have advantages in determining the relationship between ecological processes and temperature. In addition, the author collected the spatial turnover rate results of a large number of microorganisms (including bacteria, fungi, and archaea) and plants from the literature, and verified the above three hypotheses on a global scale. The results showed that, on a continental scale, the spatial turnover rates of belowground bacterial communities and microbial functional genes, the total soil microbial biomass, and the aboveground biomass of rice were all correlated with ambient temperature, consistent with the MTE theory. Through meta-analysis, the temperature dependence of the spatial turnover rates of belowground microorganisms (including bacteria, fungi, and archaea) and aboveground plants can be further extended to a global scale. In addition, belowground microorganisms affect the spatial turnover rate and ecosystem function of aboveground plants.
result
The spatial turnover rate of soil microorganisms in paddy fields conforms to the MTE theory
The spatial turnover rate of species is used to describe the replacement rate of species along the environmental gradient, which is the basis of many ecological conservation theories and practices. This study estimated the spatial turnover (z-score) of soil bacterial communities and microbial functional genes. The spatial turnover rate of underground microorganisms in 39 paddy fields in 13 regions of the country varied greatly, with the z-scores of bacteria ranging from 0.003 to 0.050 and the z-scores of microbial functional genes ranging from 0.057 to 0.091 (Fig. S2). Interestingly, no matter the bacterial community ( r 2 = 0.118, p = 0.033, AIC = -371) or microbial functional genes ( r 2 = 0.264, p < 0.001, AIC = -366) and its subgroups, the z-values significantly increased with MAT (Fig. S2). According to Pearson correlation analysis, although paddy soil properties (pH, OM, CEC, and TN) varied with MAT, they had little relationship with microbial spatial turnover (Fig. S3).
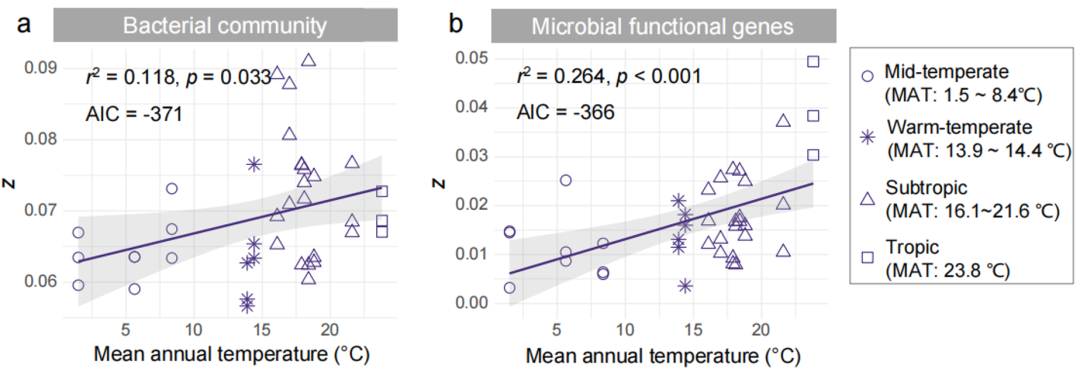
Figure S2. The relationship between the spatial turnover rate (z) of soil bacterial communities (a) and microbial functional genes (b) in 39 paddy fields in China and the annual mean temperature
The z- values of bacterial community ( r 2 = 0.118, p = 0.033, AIC = -371) and microbial functional genes ( r 2 = 0.264, p < 0.001, AIC = -366) all increased significantly with the increase of annual mean temperature . The numbers in parentheses in the figure indicate the annual average temperature range of the sampling sites. The line was fitted by least squares regression and the shaded area represents the 95% confidence limits. MAT = Mean Annual Air Temperature; AIC = Akaike Information Criterion.
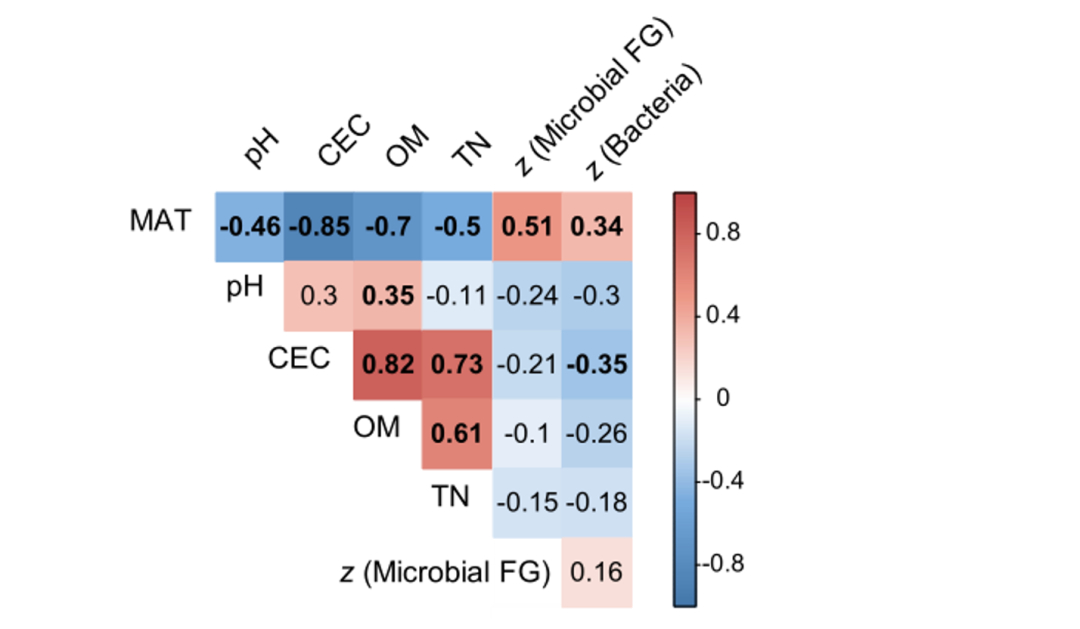
Figure S3. Correlations of environmental factors with soil bacterial communities and microbial functional gene spatial turnover (z) based on Pearson correlation analysis
Bold indicates significant correlation ( p < 0.05). MAT = mean annual temperature; CEC = cation exchange capacity; OM = organic matter; TN = total nitrogen; FG = functional gene.
To test Hypothesis 1, the researchers estimated the temperature dependence of bacterial z-scores and microbial functional gene z-scores based on MTE. The activation energy ( E ) of the underground space turnover rate is the slope of the linear regression between the ln-transformed z-value and the reciprocal of the absolute temperature (1/ kT ). Notably, we found that the log-transformed z-values for bacterial communities ( E = 0.047 eV, r = 0.128 , p = 0.025) and microbial functional genes ( E = 0.341 eV, r = 0.255 , p = 0.001) There is a strong linear relationship with 1/ k T (Fig. 2).
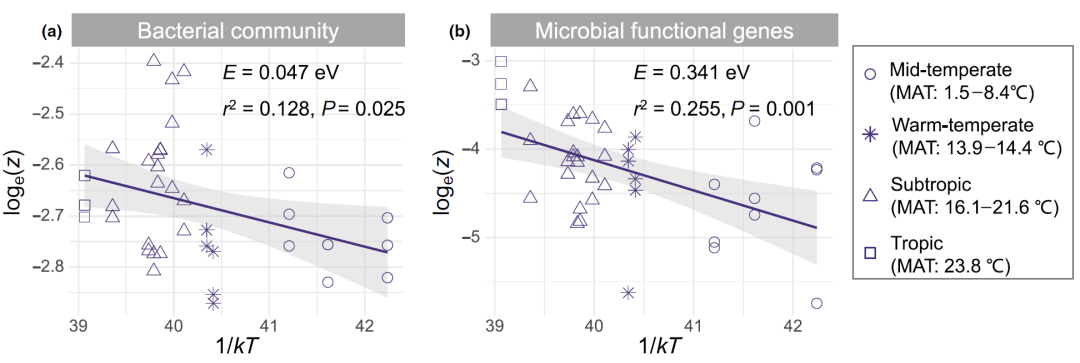
Figure 2. The temperature dependence of the spatial turnover rate (z) of bacterial communities (a) and microbial functional genes (b) in 39 paddy fields in China
According to MTE, there was a strong linear relationship between the log-transformed z-scores of bacterial communities and microbial functional genes and the inverse of absolute temperature (1/ kT ). E is the activation energy expressed as the slope of the linear regression between ln transformed z-values and 1/ kT . The numbers in parentheses in the figure indicate the mean annual temperature (MAT) interval of the sampling site. The straight line is the least squares regression fit, and the shaded area represents the 95% confidence interval.
By 999 bootstrap and paired t-tests, the E value of microbial functional genes was significantly higher than that of bacterial communities ( p < 0.01). The z-scores of bacterial subgroups also showed a strong linear relationship with 1/kT, with Bacteroidetes having the highest activation energy ( E = 0.168 eV, r 2 = 0.213, p = 0.003), followed by Actinomycetes (E = 0.120 eV, r 2 = 0.159, p = 0.012) and Chloroflexi ( E = 0.114 eV, r 2 = 0.180, p = 0.007). The z-scores of all functional gene subgroups of soil microorganisms were also consistent with MTE, and the activation energy of functional genes related to methanogenesis was the highest ( E = 0.428 eV).
Belowground microbial biomass and aboveground plant biomass
Both meet MTE
Understanding the relationship between biomass and temperature can help predict the impact of future climate change on carbon stocks. The researchers used the PLFA technique to assess the total belowground microbial biomass of paddy fields, including bacteria, fungi, and actinomycetes. The total amount of microbial PLFAs in 39 paddy fields varied from 7.49 to 50.56 nmol/g dw. Using PLFA as an indicator of microbial biomass, it was found that MAT increased the total subsurface microbial biomass, the biomass of bacteria, fungi, and actinomycetes ( r 2 = 0.161~0.221, p < 0.05; Figure S4a and c). The researchers further estimated the aboveground biomass of paddy fields based on NPP remote sensing, and the variation range was 171.95 to 521.20 g C/m 2 /year. Similarly, NPP increased significantly with MAT from the best-fit predictions, although it decreased in the tropics (exponential model: r2 = 0.246, p < 0.001) (Fig. S4b) .
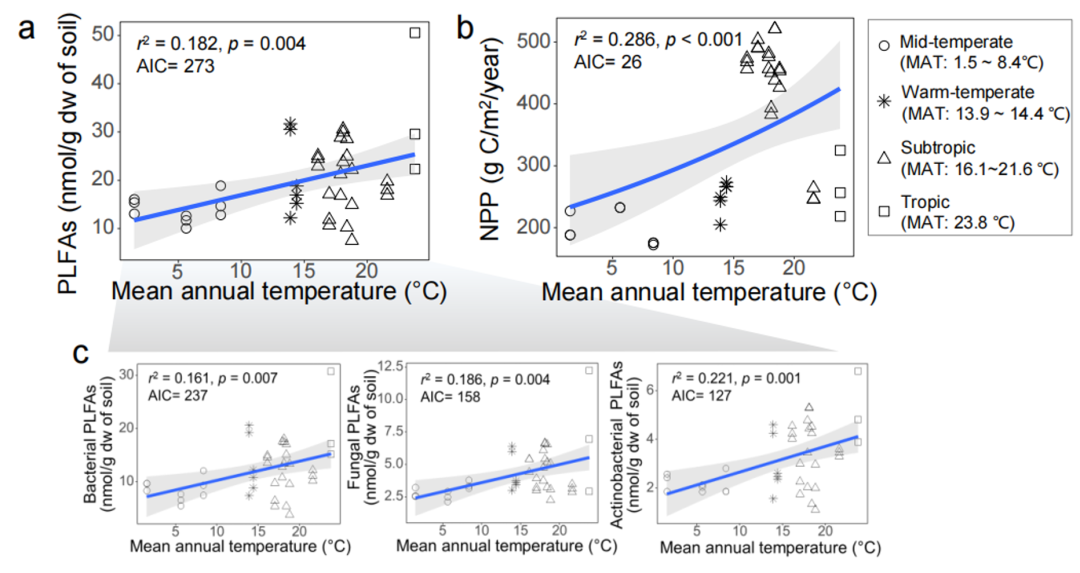
Figure S4. Relationship between annual average temperature and belowground microbial biomass (a, c) and aboveground plant biomass (b) in 39 paddy fields in China
The sum of microbial PLFAs was used as a measure of total microbial biomass, including that of bacteria, fungi, and actinomycetes. NPP is an indicator to measure the aboveground biomass of rice fields. Both microbial phospholipid fatty acids (PLFAs) and net primary productivity (NPP) increased significantly with the increase of mean annual temperature (MAT).
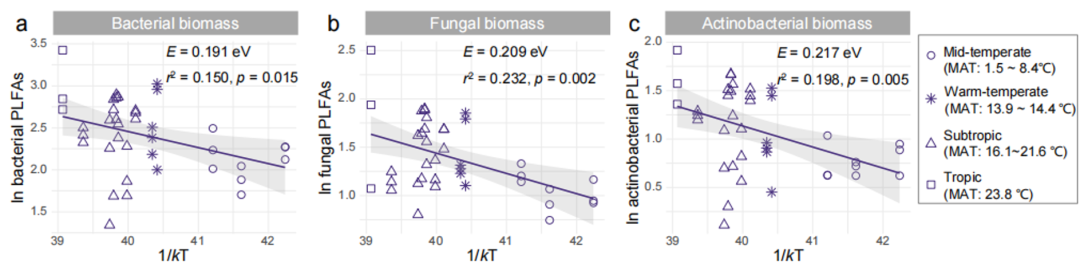
Figure S5. Temperature dependence of belowground microbial biomass in 39 paddy fields in China
(a) bacterial PLFAs; (b) fungal PLFAs; (c) actinomycetal PLFAs.
Since both PLFAs and NPPs showed a significant increase trend with the increase of MAT (Fig. S4), the researchers further explored whether the effect of temperature on biomass followed MTE. The results showed that both PLFAs and NPPs had significant temperature dependence (Fig. 3; Fig. S5). The activation energy of underground microbial PLFAs and bacteria, fungi, and actinomycetes PLFAs ranged from 0.191 to 0.217 eV ( r 2 = 0.150~0.232, p < 0.05), and that of NPP was 0.240 eV ( r 2 = 0.313, p < 0.001 ).

Figure 3. Soil microbial biomass (a) and aboveground plant biomass (b) in 39 paddy fields in China
The sum of microbial PLFAs was used as a measure of total microbial biomass, including bacteria, fungi, and actinomycetes; NPP was used as an indicator of aboveground biomass in rice fields. The effects of temperature on belowground microbial biomass and aboveground plant biomass were consistent with MTE predictions, with logarithmically transformed biomass values showing a strong linear relationship with the reciprocal of absolute temperature (1/kT). E is the activation energy, and is the slope of the linear regression between the ln transformed biomass value and 1/kT.
The relationship between the underground and aboveground parts of paddy fields
Considering that subsurface microorganisms can sustain aboveground biological life, the researchers explored how subsurface microorganisms (i.e., bacterial communities, microbial functional genes, and the spatial turnover rate of microbial biomass) are independent of temperature control over a large temperature gradient. Contribute to ecosystem functions. In this study, a random forest model was used to predict paddy field NPP using MAT, belowground microbial spatial turnover (z), and biomass (PLFAs). Taken together, these predictive factors explained 69.81% of the variance in NPP. Under a large temperature gradient, MAT was the most important factor affecting NPP in paddy fields (Fig. 4a), meanwhile, bacterial z-values also affected NPP, followed by PLFAs and microbial functional gene z-values.
Using SEMs to conduct an in-depth study of MAT, the spatial turnover of belowground species, and the direct and indirect effects of belowground biomass on aboveground biomass, the final model explained 38% of aboveground biomass variation (Fig. 4b). The results showed that MAT had the greatest direct effect on aboveground biomass production ( r = 0.53, p < 0.01). In addition, MAT also indirectly affected aboveground biomass by affecting the spatial turnover rate of bacterial communities ( r = 0.27, p < 0.05). Although the spatial turnover of belowground biomass and microbial functional genes was also regulated by temperature, its direct impact on aboveground biomass was weak. These results suggest that, in addition to temperature, spatial turnover of subsurface bacteria is also important in determining aboveground biomass, supporting Hypothesis 3.
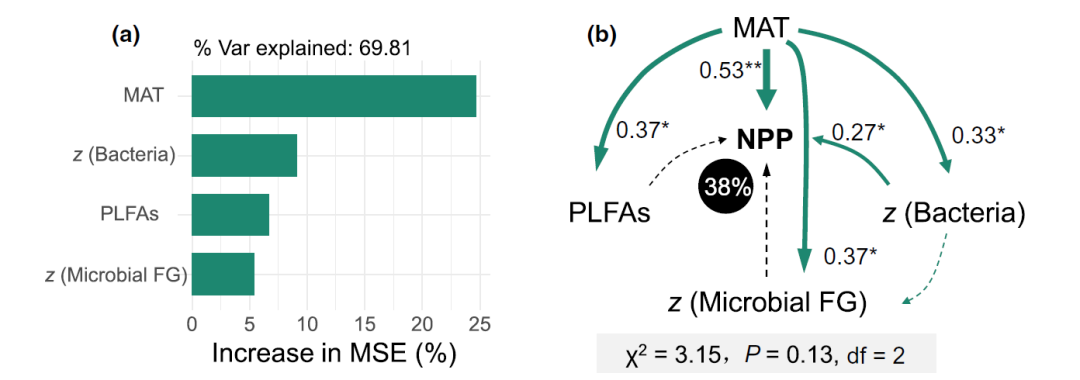
Figure 4. The relationship between the underground and aboveground parts of the paddy field
(a) Prediction of the effects of mean annual temperature, belowground microbial spatial turnover (z) and aboveground biomass on NPP based on a random forest model (MSE is the percentage increase in mean square error). (b) Structural equation modeling was used to estimate the direct and indirect effects of mean annual temperature, groundwater bacterial community and spatial turnover of microbial functional genes, and total microbial biomass on aboveground biomass. Green arrows indicate significant relationship paths ( p < 0.05); black dotted arrows indicate insignificant paths ( p > 0.05). The numbers next to the arrows are the normalized path coefficients. Numbers in black circles indicate the percentage change in aboveground biomass predicted by the model. The significance levels of each predictor are: * p < 0.05 and ** p < 0.01, respectively. MAT = mean annual temperature; PLFAs = phospholipid fatty acids; NPP = net primary productivity; FG = functional genes.
Aboveground biomass will increase soil carbon input through enhanced root exudates, thus potentially affecting belowground communities and biomass production processes. This study explored the potential impact of aboveground biomass on soil microbial biomass and spatial turnover using SEM models. Contrary to the researchers' expectations, the direct effects of aboveground biomass (i.e., paddy NPP) on belowground biomass and microbial spatial turnover in this study were weak ( p > 0.05; Fig. S6).

Figure S6. Exploring the direct and indirect effects of annual mean temperature and aboveground biomass on underground microbial spatial turnover and biomass based on structural equation modeling
Green arrows indicate significant relationship paths ( p < 0.05); black dotted arrows indicate insignificant paths ( p > 0.05). The numbers next to the arrows are the normalized path coefficients. The significance levels of each predictor are: * p < 0.05, ** p < 0.01. z(Bacteria) = spatial turnover rate of bacterial communities; z(Microbial FG) = spatial turnover rate of microbial functional genes; MAT = annual mean temperature; PLFAs = phospholipid fatty acids; NPP = net primary productivity; FG = functional genes.
Microbial and plant spatial turnover at the global scale
Meta-analysis of temperature dependence
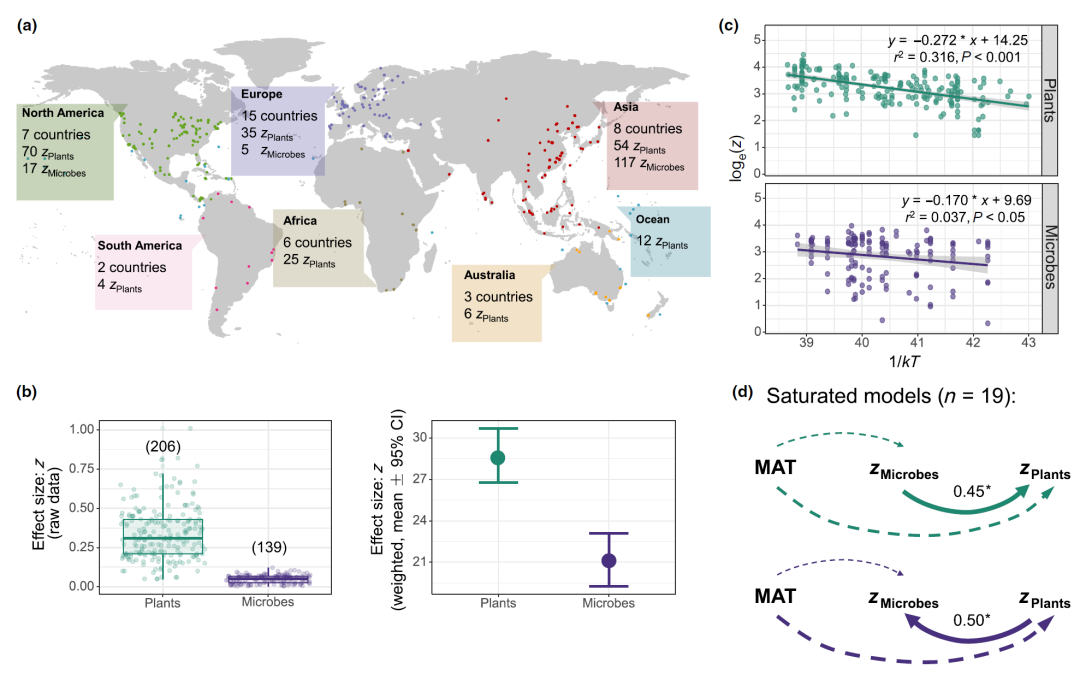
Figure 5. Global spatial turnover rate (z) of plants and microorganisms and their relationship with temperature
(a) Macroscopic plant and microbial z-score collection map. (b) z-scores for plants and microbes on a global scale. A total of 206 z-value data of plants and 139 z-value data of microorganisms (including bacteria, fungi, and archaea) were collected and correlated with the corresponding MAT data. Mean weighted z ± 95% confidence intervals (CI) are given in the figure. Boxplots showing the median (horizontal line), 25% and 75% quantiles (upper and lower bounds of the box), the most extreme values without outliers (upper and lower bounds), and outliers (samples more than 1.5 times away from the median) quantile range). (c) Global plant and microbial z-scores as a function of temperature. On a global scale, the spatial turnover rate (z) of plants and microorganisms was significantly linear with the inverse of absolute temperature (1/kT). The line represents the least squares regression fit and the shaded area represents the 95% confidence limits. (d) Using the model to relate the z-scores of microorganisms and plants obtained in the 19 data sets to adjacent geographic locations. Significance levels for predictors are as follows: * p < 0.05. M AT = mean annual air temperature. The z-scores of plants and microorganisms in adjacent geographic locations were linked using 19 sets of data in the SEM model. Significance levels for predictors are as follows: * p < 0.05. MAT = mean annual air temperature.
Given the strong temperature dependence of the spatial turnover rate of species and the interaction between microorganisms and plants (Fig. 2-4), the goal of this study was to obtain a unified explanation of the global spatial turnover patterns of microorganisms and plants. Spatial turnover z-scores for all species (including macroscopic plants and microbes) available in the literature and in this study were considered effect sizes (Fig. 5). A total of 465 plant z-scores and 145 microbial z-scores were obtained, including bacteria, fungi, and archaea. Among these data, some studies can infer the MAT value of the region directly from the original paper or according to the latitude and longitude coordinates (plants: n = 206; microbes: n = 139), including 7 countries in North America, 2 countries in South America, 15 countries in Europe, 6 countries in Africa, 8 countries in Asia, 3 countries in Australia, and other marine sites (Fig. 5a). Raw data and weighted z-score distributions for plants and microbes are shown in Fig. 5b. Figure 5c fits the z value and 1/kT with linear regression, and the results show that, on a global scale, plant ( E = 0.272 eV, p < 0.001) and microorganism ( E = 0.170 eV, p < 0.05) z values are correlated with mean There was a significant linear relationship between 1/ k T (Fig. 5c), with a very low r2 value (0.037) for microorganisms. Using the saturated SEMs model to correlate 19 groups of adjacent microbial and plant z-values (Fig. 5d), it was found that microbial z-values significantly affected plant z-value changes (r = 0.45 , p < 0.05). In addition, this study also found that even when MAT was controlled, the feedback effect of plants to microbes still existed ( r = 0.50, p < 0.05).
discuss
The spatial turnover rate of biodiversity is used to describe the replacement rate of species along the environmental gradient, which is the basis of many environmental protection theories and practices. Although microorganisms are a major component of Earth's biodiversity, the temperature dependence of microbial spatial turnover rates has been poorly studied in the context of current global warming. Based on the MTE theory, the researchers used data from 39 rice fields in China to investigate the temperature dependence of the spatial turnover rate of soil bacterial communities and microbial functional genes. The results of the study showed that the spatial turnover rate of microorganisms in paddy soil exhibited temperature dependence and was consistent with MTE (hypothesis 1, Fig. 2). The meta-analysis found that this conclusion also applies to microorganisms on a global scale, including a wide range of microbial groups (such as bacteria, fungi, and archaea) and habitats (Fig. 5). These findings, combined with previous studies showing that forest soil microbial diversity was MTE-compliant, showed that both native species richness (α-diversity) and spatial turnover (β-diversity) increased with increasing ambient temperature.
The activation energies of bacterial community and microbial functional gene space replacement were 0.047 eV and 0.341 eV, respectively. Previous studies have shown that E-values increase with taxonomic/genetic resolution, thus spatial turnover of microbial functional genes increases faster with temperature than bacterial communities, most likely because of functional gene resolution higher than the species. The results of this study, together with previous studies, suggest that the most diverse microbes may have E-values for both alpha and beta diversity below the previously predicted range of 0.60–0.70 eV. For reference, the E value is denoted by Q10, which is defined as the rate of change in diversity per 10°C increase in temperature. The bacterial E value was 0.047 eV, and the functional gene E value was 0.341 eV. The corresponding Q10 values of the two were 1.07 and 1.64, respectively, that is, the spatial turnover rate of the soil microbial community increased by 1.1 to 1.6 times for every 10°C increase in the annual average temperature. In addition, meta-analysis showed that the spatial turnover of plants worldwide also conformed to MTE; and the temperature dependence of microbial spatial turnover rate ( E = 0.170 eV) was lower than that of plants ( E = 0.272 eV), which was consistent with α-diversity. The lower temperature dependence of microorganisms may be partly due to their greater dispersal ability and shorter generation time.
Based on the data set of 39 paddy fields, the researchers found that the second hypothesis of this study was also confirmed, that is, both belowground microbial biomass and aboveground biomass reflecting ecosystem processes were consistent with MTE (Fig. 3). The temperature dependence of aboveground plant biomass is consistent with previous observations for terrestrial NPP. The temperature dependence of NPP was mainly directly affected by temperature and indirectly affected by the length of non-growing season and solar radiation. It is worth noting that NPP decreases in the tropics, that is to say, the temperature here is higher than the optimum growth temperature (22-28°C). The temperature dependence of aboveground plant biomass ( E = 0.240 eV) observed in this study was lower than that of previously reported terrestrial ecosystem plant biomass ( E = 0.320 eV), which may be related to frequent human activities (such as plowing) during rice cultivation. related to fertilization), which may flatten the Boltzmann-Arrhenius curve. Furthermore, this study demonstrates for the first time that temperature-dependent subsurface microbial biomass follows the MTE ( E = 0.198 eV). However, the temperature dependence of belowground microbial biomass was lower than that of aboveground plant biomass, a result contrary to the researchers' expectations. Previous studies have shown that microbial biomass, which is mainly regulated by respiration, should have a stronger temperature dependence than NPP, which is mainly regulated by photosynthetic rate. This difference may be caused by the method of microbial biomass measurement in this study, because soil type may have a significant impact on the quantity and morphology of PLFA. More standard assays such as chloroform fumigation extraction could complement this study.
By linking below- and above-ground biota and community-level characteristics (i.e., spatial turnover rates) to ecosystem processes (i.e., biomass production), the results of a meta-analysis based on 39 paddy field datasets corroborate this study. Three hypotheses, that is, the mutual feedback between aboveground plants and belowground microorganisms (Fig. 4, Fig. 5). It is widely accepted that vegetation change is strongly influenced by soil biota, and that soil biota has feedback effects on plant growth, especially at local spatial scales. This study extends the relationship between the two to a global scale, and the results show that even when the ambient temperature is controlled, there is still a significant correlation between the above-ground and underground space turnover rates, which indicates that the two space turnover rates are not only related to MAT , other factors also contribute to the association, which may include shared evolutionary history, plant species-specific symbiosis, and rhizosphere carbon deposition. It should be noted that since the data sets used in the meta-analysis covered a wide range of habitats and were obtained by different experimental methods, it can be seen from the significant but low correlation between temperature and microbial spatial turnover , the results of this study may only roughly reflect the global distribution pattern of community spatial turnover. Interestingly, a dataset of 39 paddy fields showed a causal relationship between belowground spatial turnover and aboveground biomass, suggesting that temperature-driven spatial heterogeneity in microbial diversity may be directly through various biotic interactions, or indirectly through Potential effects of shifts in soil nutrient availability and predation by herbivores on plant growth. The lack of coupling between above-ground and below-ground biomass suggests that the mechanisms governing the biomass accumulation process are different.
In general, this study combined the data sets of 39 rice fields in China and the global meta data sets to verify the MTE theory for the process from underground to aboveground, from community to ecosystem. For belowground microorganisms and aboveground macroscopic plants, the spatial turnover rate of ecological organization at the community level and the distribution of biomass accumulation at the ecosystem level were in line with MTE. Belowground community processes have profound effects on aboveground community dynamics and ecosystem function, while aboveground community dynamics have feedback effects on belowground community processes. This study has important implications for MTE and aboveground-subsurface ecology under future climate change scenarios. Meanwhile, this study also found that MTE can be used to study many phenomena in biology, although our results together with several previous reports support an emerging theory that there is no standard ET value of ~0.65 eV. Due to the strong temperature dependence and close aboveground-subsurface connection of microbial spatial turnover, when the biological habitat area is reduced, climate warming will promote the vulnerability of aboveground and belowground biodiversity, thereby accelerating the accumulation of ecosystem biomass.
references
Xian Xiao, Zhiyuan Ma, Jiabao Zhang, Bo Sun, Jizhong Zhou & Yuting Liang (2023). Coupling temperature‐dependent spatial turnover of microbes and plants using the metabolic theory of ecology. New Phytologist, 238: 383-392.
About the Author

First author
Xiao Xian
Associate Professor, Nanjing Institute of Soil Science, Chinese Academy of Sciences
Mainly engaged in the research of soil microbial ecology and material cycle and soil microbial remediation technology. Published 16 SCI papers and 5 Chinese core papers. Presided over 1 project of the National Natural Science Foundation of China Youth Science Fund Project, and 1 general project of natural science research in colleges and universities in Jiangsu Province.

Corresponding Author
Liang Yuting
Researcher at Nanjing Institute of Soil Science, Chinese Academy of Sciences
Mainly engaged in research in the field of soil microbiology. Published more than 70 SCI papers and more than 10 Chinese core papers. Undertake the National Natural Science Excellent Youth Fund, Jiangsu Provincial Outstanding Youth Science Fund, etc., outstanding member of the Youth Promotion Association of the Chinese Academy of Sciences, serve as editorial board members of "Journal of Soil Science", Soil Ecology Letters, SBB, Geoderma, etc., director of the Soil Quality Standardization Committee of the Soil Society of China, Jiangsu Director of the Academic Working Committee of the Provincial Soil Society.
more recommendations
(▼ Click to jump)
iMeta | A collection of high-value drawing website imageGP+ video tutorials
1 volume 1 issue
Volume 1 Issue 2
Volume 1 Issue 3
1 volume 4 issues
2 volumes 1 issue
Journal Profile
"iMeta" is an open-access journal co-published by Wiley, the Enterobacteriaceae Branch and hundreds of Chinese scientists in this field. The chief editors are Researcher Liu Shuangjiang from the Institute of Microbiology, Chinese Academy of Sciences and Professor Fu Jingyuan from the University of Groningen in the Netherlands. The aim is to publish original research, methods and reviews for the advancement of metagenomics, microbiome and bioinformatics. The goal is to publish high-impact papers in the top 10% (IF > 15). The features of the journal include video submission, reproducible analysis, picture polishing, young editorial board members, free publication fee for the first 3 years, social media promotion of 500,000 users, etc. It will be officially published in February 2022!
contact us
iMeta homepage: http://www.imeta.science
Publisher: https://onlinelibrary.wiley.com/journal/2770596x
Submission: https://mc.manuscriptcentral.com/imeta
Email: [email protected]