I. Introduction
Intracranial hemorrhage (ICH) is an intracranial bleeding disease caused by a variety of reasons, including spontaneous bleeding and secondary bleeding caused by trauma. The diagnosis and treatment involve neurosurgery, neurology, critical care medicine, rehabilitation, etc. Multiple disciplines are an important challenge for clinicians. How to find patterns in the complex clinical manifestations, signs and auxiliary examinations of diseases, and then understand the diseases, is a concern of clinicians. In recent years, the advancement of artificial intelligence (AI) technology represented by deep learning has provided us with new ways to understand diseases. This article briefly sorts out the terminology of artificial intelligence and reviews previous related research, hoping to help clinicians gain a deeper understanding of the role of artificial intelligence in the diagnosis and treatment of intracranial hemorrhage. The concept of "artificial intelligence" was proposed in the 1950s, which refers to intelligence realized through artificial methods based on the understanding of intelligence (such as learning, reasoning, thinking, planning, etc.) [1]. Classic artificial intelligence mainly uses various algorithms to learn patterns in data, especially classification patterns. The main method is machine learning (ML). Classic algorithms often do not perform feature transformation, or only perform feature transformation or selection once, that is, shallow learning methods, including linear discriminant analysis (LDA), decision tree (DT), support vector machine (SVM) and naive Bayan for classification. Yessian (NB), as well as K nearest neighbor (KNN), logistic regression analysis, etc. Each algorithm has its own limitations. In order to overcome the shortcomings of classic algorithms, computer scientists have proposed deep learning methods based on multiple transformations of features, which have become a hot topic in machine learning in recent years. The basis of deep learning is artificial neural network (ANN, hereinafter referred to as neural network). Through multi-layer neural network and back propagation (BP) algorithm, different network structures are established, including autoencoder and restricted Boltzmann machine. (RBM), convolutional neural network (CNN), recurrent neural network (RNN), etc. [2]. Clinicians have long hoped to use artificial intelligence technology to improve the diagnosis and treatment of intracranial hemorrhage. As early as the 1980s when CT had not yet become popular in universities, Panzer et al. [3] from the University of Rochester School of Medicine in the United States reported a computer-aided decision-making system to assist in the diagnosis of cerebral hemorrhage based on the patient's clinical symptoms and signs. Discriminant analysis and Naive Bayes theory use plain head CT scan as the "gold standard" for diagnosing cerebral hemorrhage, and its diagnosis is accurate.
The rate is only 5% ~ 67%. In 1995, Phillips et al. [4] developed an algorithm for automatically segmenting intratumoral hemorrhage in brain glioblastoma. Based on the head MRI image of a patient with brain glioma, they used unsupervised fuzzy C-means (FCM) clustering. This class algorithm uses imaging and pathology as the "gold standard" to achieve automatic segmentation of hematoma. In 1998, Zernikow et al. [5] reported a model that used clinical information to predict intraventricular hemorrhage in premature infants. With the help of neural network algorithm, the model finally achieved the area under the receiver operating characteristic curve (ROC) curve (AUC) in the validation set. is 0.94, which is better than the model constructed with logistic regression analysis (AUC value 0.88). The following year, Edwards et al. [6] discussed the application of neural network algorithms in the prognosis of intracranial hemorrhage. This study included a total of 81 patients with supratentorial hemorrhage. Since no validation set and test set were set, only their results in the training set were reported. The death prediction accuracy is 100%, which is better than the 79% of logistic regression analysis. Although the above-mentioned research methods have various problems and the results are unsatisfactory according to current standards, as early as the end of the 20th century, researchers have demonstrated several important directions for the application of artificial intelligence in intracranial hemorrhage, namely clinical decision support systems. (diagnosis, treatment, prognosis) and neuroimaging analysis, while also demonstrating the potential of neural network algorithms.
2. Application of artificial intelligence in the diagnosis of intracranial hemorrhage
To date, many studies have used machine learning methods to automatically identify intracranial hemorrhage in various types of neuroimaging. In 2018, Chilamkurthy et al. [7] published a new algorithm in Lancet and achieved relatively accurate results. This algorithm can determine 5 different types of intracranial hemorrhage, while identifying skull fractures and midline shifts. 20 medical centers were included 313 318 From the head CT images of patients with intracranial hemorrhage, 23,263 cases were randomly selected as the verification set, and the remaining 290,055 cases were used as the training set. At the same time, 491 head CT images of patients with intracranial hemorrhage were collected as the test set. The model uses a deep learning method and uses the independent judgment of three radiologists as the diagnostic standard. Finally, the algorithm diagnoses intracranial hemorrhage, cerebral hemorrhage, intraventricular rupture, subdural hemorrhage, epidural hemorrhage and subarachnoid hemorrhage in the validation set. The AUC values of the ROC curve for intracavitary hemorrhage are 0.92, 0.90, 0.96, 0.92, 0.93 and 0.90 respectively, and the AUC values in the test set are 0.94, 0.95, 0.93, 0.95, 0.97 and 0.96 respectively; this algorithm diagnoses skull fractures and midline shifts And the effect of placeholder effect is also good, its AUC values in the validation set are 0.92, 0.93 and 0.86 respectively, and in the test set the AUC values are 0.96, 0.97 and 0.92 respectively [7]. Since then, scholars have continued to try to improve training efficiency by improving algorithms. In 2019, Ye et al. [8] tried a new deep learning architecture, using the structure of a three-dimensional convolutional neural network and a series of recurrent neural networks to obtain data from fewer plain head CT scan images (1836 cases of cerebral hemorrhage and 1000 cases of normal controls). Similar results were obtained with Chilamkurthy et al. [7]. The AUC value of the ROC curve for diagnosing cerebral hemorrhage in the validation set of this algorithm was ≥ 0.98, and the AUC value for judging 5 subtypes of cerebral hemorrhage was > 0.80, and it was confirmed that the algorithm is better than the ones being trained. Manual diagnosis by junior radiologists. In the same year, Ker et al. [9] tried to improve the training efficiency of the convolutional neural network algorithm by performing threshold conversion (thresholding) preprocessing on CT images. They were about to determine the cause of intracranial hemorrhage through only the head CT scan images of 399 patients. F1 score increased from 0.71 ~ 0.90 to 0.93 ~ 0. 95. In addition, some scholars continue to explore algorithms that are closer to the real world. In 2017, Prevedello et al [10] demonstrated an algorithm for judging intracranial hemorrhage in the case of various intracranial lesions, including 100 cases of intracranial lesions including cerebral hemorrhage, intracranial space-occupying lesions and hydrocephalus, 22 cases of acute ischemic stroke and 124 normal controls, based on head CT plain scan images, with the help of convolutional neural network algorithm, respectively trained with brain tissue window and stroke window. Finally, the algorithm diagnosed intracranial lesions in the test set. The AUC value of the tissue window ROC curve was 0.91, and the AUC value of the stroke window was 0.81. In 2016, Qi et al. [11] built an automatic recognition model for cerebral microbleeds (CBMs). A total of 320 head MRI images of patients with cerebral microbleeds were concatenated with the help of series The three-dimensional convolutional neural network algorithm has a final diagnostic sensitivity of 93%.
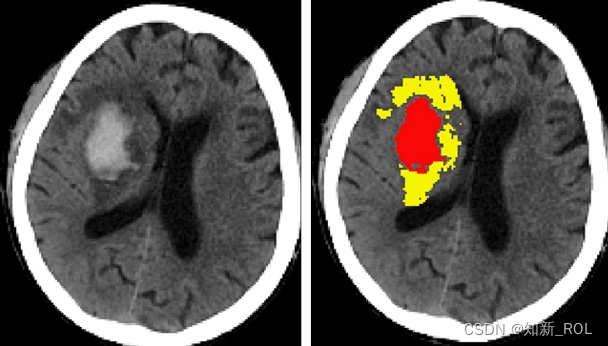
3. Application of artificial intelligence in intracranial hematoma segmentation
The bleeding volume, bleeding site and bleeding time of intracranial hemorrhage are important factors affecting treatment decisions and prognosis. Whether hematoma can be accurately segmented on images is the first step to use artificial intelligence technology for more in-depth analysis. There are currently many studies exploring various automatic segmentation methods, but no breakthrough progress has been made yet. Such studies mainly use manual segmentation of hematomas by imaging experts as the "gold standard" for evaluating algorithmic segmentation. In 2018, Chang et al. [15] reported an algorithm that can roughly identify the bleeding site in head CT images. Using the bounding boxes regression method, a hybrid three-dimensional and/or two-dimensional template based on the region of interest (ROI) was used. Assess hematoma, and then use the convolutional neural network algorithm to perform deep learning. The head CT scan images of 10,159 patients with intracranial hemorrhage were used as a training set. Finally, the ROC curve AUC value of the algorithm for diagnosing intracranial hemorrhage in the test set was 0.98, and the recognition The Dice values of cerebral hemorrhage, subdural hemorrhage and/or epidural hemorrhage, and subarachnoid hemorrhage are 0.93, 0.86, and 0.77 respectively, but the algorithm cannot automatically mark the hematoma boundary and calculate the hematoma volume. In 2016, Scherer et al. [16] from Heidelberg University Hospital in Germany published an algorithm in Stroke that can directly segment hematomas in head CT scan images. The 58 patients with cerebral hemorrhage included included 28 in the training set and 30 in the validation set. For example, using the voxel-based random forest method, two researchers independently manually segmented hematoma, brain tissue, and subarachnoid hemorrhage. In the final validation set, the consistency correlation coefficient (CCC) between the algorithm and manual segmentation was 0.99. The consistency correlation coefficient with Tada's formula is 0. 82. Although the hematoma volume calculated by Tada's formula is significantly larger than that of manual segmentation, the difference between the three is not statistically significant. In 2019, Cho et al. [17] used a new deep learning framework to report an algorithm that can more accurately identify and automatically segment hematomas. With the help of convolutional neural networks and fully convolutional networks (FCN), 135,974 patients with intracranial hemorrhage were analyzed. The brain tissue window and stroke window of the head CT plain scan image are automatically segmented. Finally, the segmentation effect can be improved by connecting two convolutional neural networks and one fully convolutional network in series, making the segmentation accuracy reach 80% and the regression rate reach 82%. . Some scholars also tried to improve the accuracy of segmentation through head MRI images. Morrison et al. [18] described a semi-automatic segmentation algorithm for cerebral microbleeds. They automatically segmented hematomas and manually corrected them. Finally, in the test set, the algorithm was the same as manual segmentation. The consistency correlation coefficient reaches 0.97.
4. Application of artificial intelligence in predicting the progression of intracranial hemorrhage
Hematoma expansion and secondary ischemic stroke after intracranial hemorrhage are also issues that clinicians are concerned about, and there are currently few relevant studies. Tan et al [19] explored an algorithm to automatically predict the progression of hematoma based on the "spot sign". This study included 42 patients with cerebral hemorrhage, based on dual-source CT enhanced images, using naive Bayes theory for machine learning, and automatically Identify characteristics such as contrast agent extravasation, and finally discover two imaging features (total iodine content in the hematoma and local iodine content in the brightest part of the hematoma) and establish a new scoring system. The sensitivity and specificity of this algorithm in the test set are both higher than Manual identification. Tanioka et al [20] reported a model for predicting delayed ischemic stroke after intracranial hemorrhage, analyzing 12 clinical variables and expression changes of serum cellular matrix proteins (MCPs) in 95 patients with aneurysmal subarachnoid hemorrhage (aSAH). , using the random forest method to build a prediction model, the final prediction accuracy reached 95%. The important influencing factors of this algorithm are the expression levels of three cell matrix proteins and the location of intracranial aneurysms. Ramos et al [21] combined clinical variables and head CT scan images to construct a model for predicting ischemic stroke. They used minimum redundancy maximum correlation (mRMR), support vector machine and partial least squares regression for machine learning and found that 317 For a patient with aneurysmal subarachnoid hemorrhage, the AUC value of the ROC curve of the model that included CT scan image information was 0.74, which was higher than the model built solely on clinical information (AUC value was 0.68). Park et al. [22] used an algorithm similar to Ramos et al. [21] and combined clinical information with vital signs and other indicators. Finally, the AUC value of the ROC curve of the algorithm in the validation set was 0.77. Machine learning methods are also used to predict the rupture risk of micro-aneurysms. Kim et al. [23] used three-dimensional digital subtraction angiography (3D-DSA) to automatically identify aneurysms and build a prediction model. A total of 368 patients with micro-aneurysms were included. With the help of the convolutional neural network algorithm, the model finally predicted the rupture of small aneurysms with an accuracy of 77% in the test set.
5. Application of artificial intelligence in predicting the prognosis of intracranial hemorrhage
Predicting the outcome of intracranial hemorrhage has always been a concern for clinicians. Traditional analysis methods have been used to construct some scores and scales that can predict prognosis. With the development of artificial intelligence technology, it is possible to process a large amount of clinical data and construct More accurate models are possible. Gupta et al [24] constructed an intracerebral hemorrhage outcome model (ICHOP) to predict modified Rankin Scale (mRS) scores 3 and 12 months after discharge, collected more than 200 variables from 575 patients with cerebral hemorrhage, and screened related factors based on the random forest method. A prediction model was constructed using linear regression. The AUC values of the ROC curve for predicting mRS scores 3 and 12 months after discharge were 0.89 and 0.87 respectively, which were better than the traditional cerebral hemorrhage score (AUC values 0.84 and 0.81). Zafar et al [25] demonstrated their Glasgow prognostic classification (GOS) model for predicting patients with aneurysmal subarachnoid hemorrhage, including a total of 153 patients with 473 variables, including clinical characteristics, physiological parameters, etc., using logistic regression analysis and multivariate multivariate analysis. The horizontal method was used to construct a prediction model, and the ROC curve AUC values of the model for predicting death and independent life were 0.92 and 0.95, respectively. Rohaut et al. [26] reported a model for predicting the short-term recovery of consciousness in patients with cerebral hemorrhage. Among the 158 patients with cerebral hemorrhage, 105 had good consciousness and 53 had lost consciousness. The head MRI images of all subjects were collected and analyzed through elastic network. Logistic regression analysis was used to construct a prediction model. The AUC value of the ROC curve for predicting the state of consciousness when leaving the intensive care unit was 0.74, and the AUC value for judging the state of consciousness when undergoing MRI scanning through MRI images was also 0.74.
6. Some references
[1] Wang RZ, Feng M, Liu XH. Applying artificial intelligence technology promote the development of neurosurgery[J]. Zhongguo
[2] Wei Qin Xi Shen Jing Wai Ke Za Zhi, 2018, 23:241⁃243[. Wang Renzhi, Feng Ming, Liu Xiaohai. Using artificial intelligence technology to promote the development of neurosurgery [J]. Chinese Journal of Microinvasive Neurosurgery, 2018, 23:241⁃243.] [3] Hu Y, Luo DY, Hua K, Lu HM
, Zhang XG. Overview on deep learning[J]. Zhi Neng Xi Tong Xue Bao, 2019, 14:1⁃19[. Hu Yue, Luo Dongyang, Hua Kui, Lu Haiming, Zhang Xuegong. Overview and discussion on deep learning[J ]. Journal of Intelligent Systems, 2019, 14:1⁃19.]
[4] Panzer RJ, Feibel JH, Barker WH, Griner PF. Predicting the likelihood of hemorrhage in patients with stroke[J]. Arch Intern Med, 1985, 145 :1800⁃1803.
[5] Phillips WE 2nd, Velthuizen RP, Phuphanich S, Hall LO, Clarke LP, Silbiger ML. Application of fuzzy C ⁃ means segmentation technique for tissue differentiation in MR images of a hemorrhagic glioblastoma multiforme[J]. Magn Reson Imaging, 1995, 13:277⁃290.
[6] Zernikow B, Holtmannspoetter K, Michel E, Theilhaber M,Pielemeier W, Hennecke KH. Artificial neural network for predicting intracranial haemorrhage in preterm neonates[J]. Acta Paediatr, 1998, 87:969⁃975.
[7] Edwards DF, Hollingsworth H, Zazulia AR, Diringer MN. Artificial neural networks improve the prediction of mortality inintracerebralhemorrhage[J]. Neurology, 1999, 53:351⁃357.