
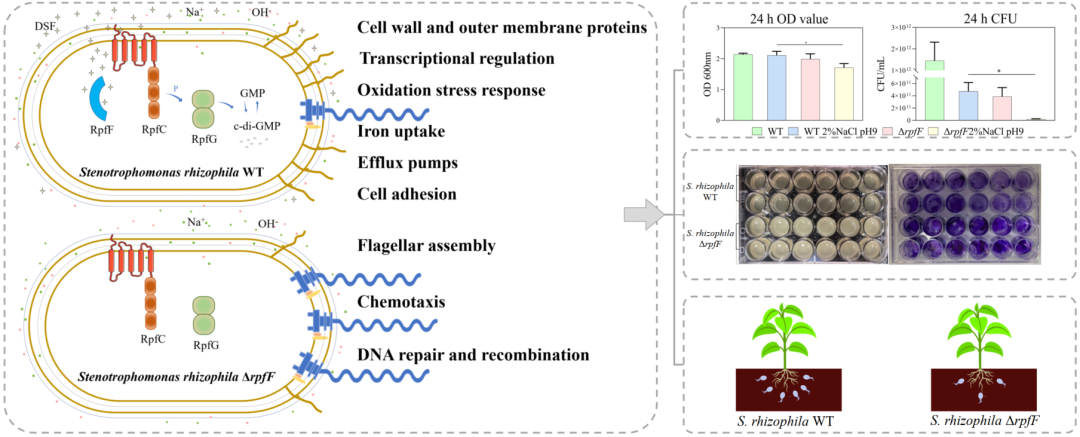
图文摘要 | Graphical Abstract
导读 | Introduction
随着土壤盐碱化日益严重,应用植物根际促生菌这一生物防治手段,来帮助植物应对盐碱胁迫愈受重视,作为植物根际促生菌的嗜根寡养单胞菌可以提高植物胁迫耐受能力,具有广阔的应用前景。群体感应(Quorum sensing,QS)作为细菌间信号传导与交流的重要机制,在植物根际促生菌发挥功能的过程中起着重要作用,其中,可扩散性信号分子(Diffusible signal factor,DSF)是一种广泛存在的QS信号分子。嗜根寡养单胞菌具有负责DSF-QS调控的rpf基因簇。因此,本研究利用可产生DSF的嗜根寡养单胞菌野生型和无法分泌DSF的rpfF基因敲除株,探究DSF-QS在植物根际促生菌中的作用。今天,让我们来看一下DSF-QS是如何帮助嗜根寡养单胞菌抵抗盐碱胁迫的吧?
As soil salinization becomes more and more serious, the application of plant growth-promoting rhizobacteria (PGPR) to help plants cope with saline-alkaline stress has been paid more attention. Stenotrophomonas rhizophila as PGPR can improve the stress tolerance of plants and thus has broad application prospects. PGPR plays a growth-promoting role in the process of rhizosphere colonization, which can not be separated from the participation of quorum sensing (QS). QS is the mechanism of microbial communication through the secretion and perception of signal molecules. Diffusible signal factor (DSF) is a ubiquitous QS signaling molecule, and S. rhizophila has the rpf gene cluster responsible for DSF-QS regulation. In this study, we used S. rhizophila wild-type (WT) and an incompetent DSF production rpfF-knockout mutant (ΔrpfF) to explore the regulatory role of QS in S. rhizophila growth, stress responses, biofilm formation, and colonization under saline-alkaline stress. Today, let us look at how DSF-QS can help S. rhizophila resistant to saline stress.
一、DSF-QS调控嗜根寡养单胞菌代谢活动与种群增殖平衡
DSF-QS regulates the balance of metabolic activity and population proliferation of S. rhizophila
通过比较标准培养与盐碱胁迫下野生型和rpfF基因敲除株的生长情况(图1)发现,盐碱胁迫显著抑制了rpfF基因敲除株的生长;24小时后,rpfF基因敲除株的细胞数量约为野生型的1/25。那么,这种现象是什么原因造成的呢?为此,本研究分别获取了培养24小时状态下,标准培养野生型(Sr)、盐碱胁迫野生型(SrS)和盐碱胁迫rpfF基因敲除株(SrM)的mRNA,进行转录组测序以探究其分子机制。
By comparing the growth of WT and ΔrpfF in standard and saline-alkaline stress cultures (Fig. 1), saline-alkaline stress significantly inhibited the growth of ΔrpfF compared with WT, and the number of bacteria in ΔrpfF was about 1/25 of that in WT after 24 hours. So, what is the cause of this phenomenon? In this study, the mRNA of WT in standard LB medium (Sr group), WT in saline-alkaline LB medium (SrS group), and ΔrpfF in saline-alkaline LB medium (SrM group) were obtained after 24 hours of culture, and transcriptome sequencing was carried out to explore the molecular mechanism.
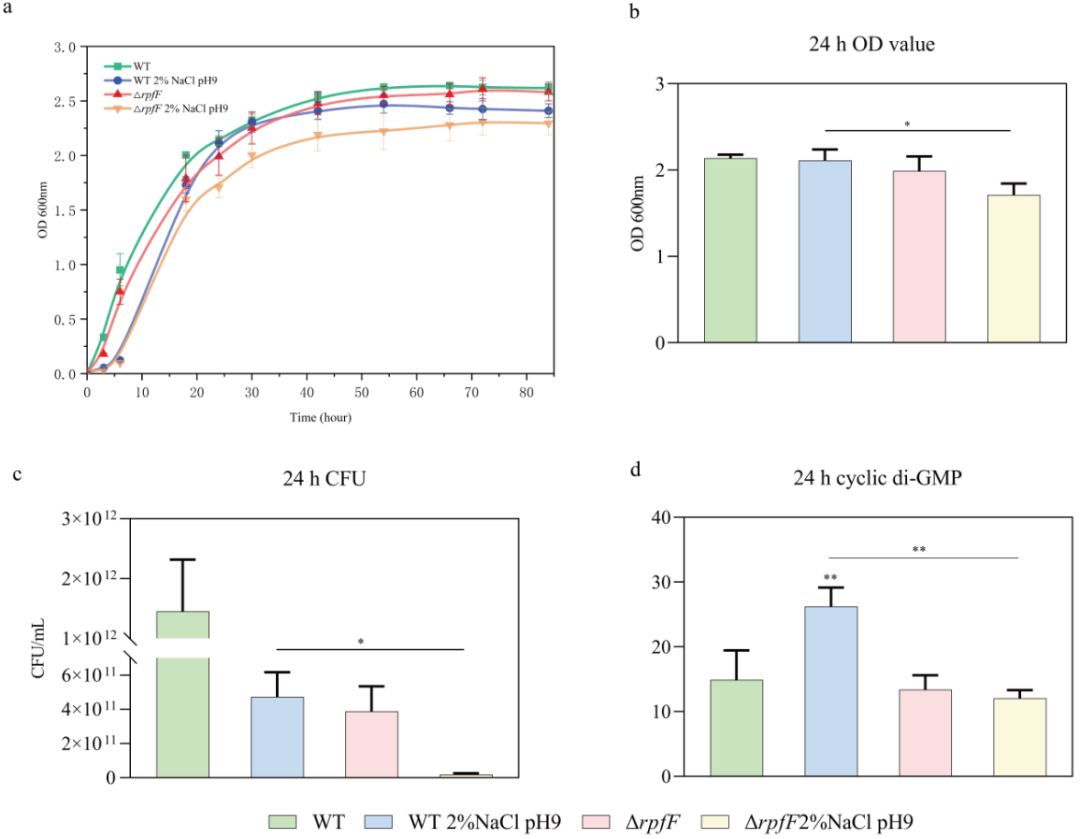
图1 标准与盐碱胁迫生长条件下嗜根寡养单胞菌野生型和rpfF基因敲除株生长状态的对比
Fig. 1 Comparison of growth status of S. rhizophila WT and ΔrpfF under standard and saline-alkaline stress conditions
数据分析发现,野生型和rpfF基因敲除株对盐碱胁迫的反应机制相似,但部分代谢活动差异导致了两者抗盐碱胁迫的差异。如图2所示,DSF-QS促进野生型细胞壁和细胞膜生成、氧化应激反应、细胞黏附、分泌系统、外排泵系统、TonB系统、抗生素合成、金属肽酶活性、信号转导和DNA复制等相关代谢活动的基因转录,以增强细胞渗透防御、钠离子外排、铁摄取和清除活性氧等能力,从而调控细胞个体代谢活动,抵抗盐碱胁迫,维持细菌种群密度。
Transcriptome data revealed that WT and ΔrpfF had similar response mechanisms to saline-alkaline stress. However, the difference in transcriptional expression of some metabolic activities led to the difference in saline-alkaline stress resistance between WT and ΔrpfF. As shown in Fig. 2, DSF-QS promoted transcription of genes involved in metabolic activities such as the cell wall and membrane formation, oxidative stress response, cell adhesion, secretion systems, efflux pump systems, TonB system, antibiotic synthesis, metallopeptidase activity, signal transduction, and DNA replication. These metabolic activities enhance the ability of bacterial osmotic defense, Na+ efflux, iron uptake, and ROS scavenging. Thereby DSF-QS regulates the metabolic activity of individual cells, resisting saline-alkali stress and maintaining bacterial population density.
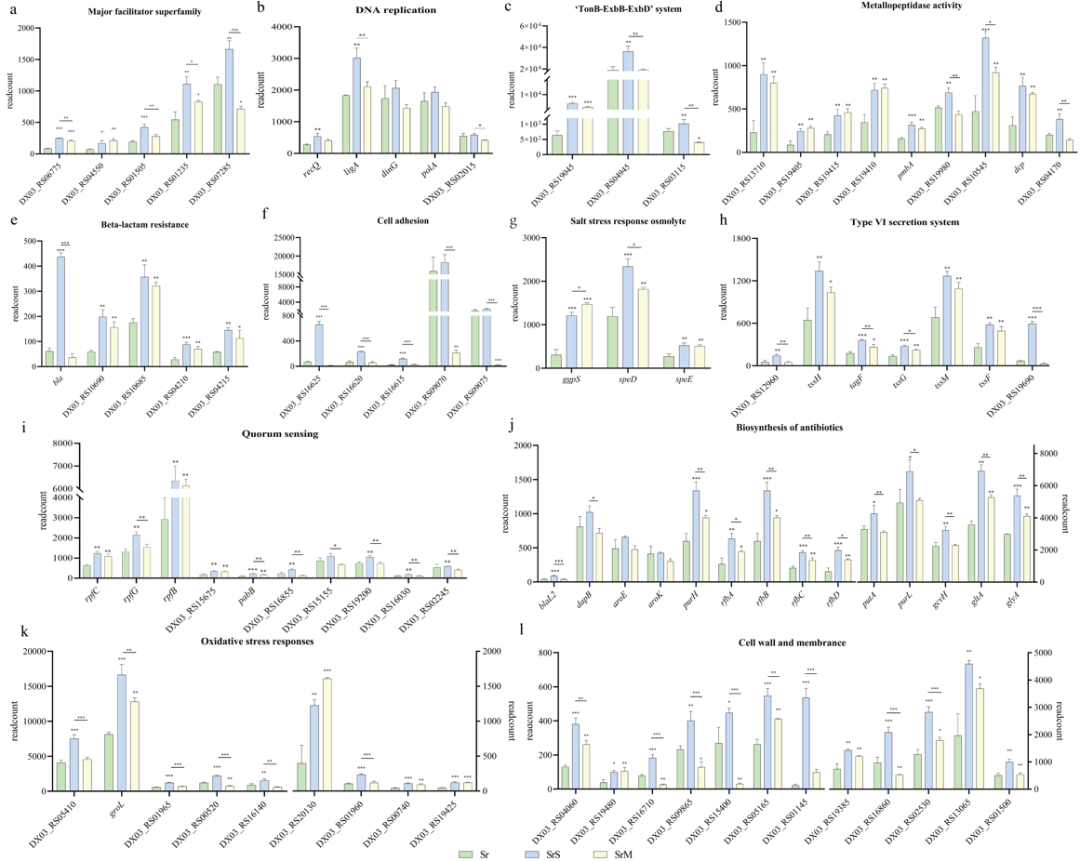
图2 标准培养野生型、盐碱胁迫野生型和盐碱胁迫rpfF基因敲除株代谢活动差异表达基因的比较-1
Fig. 2 Comparison of differential expressed genes (DEGs) in metabolic activities of Sr, SrS and SrM groups-1
二、DSF-QS缺失导致rpfF基因敲除株生物被膜过表达
Deletion of DSF-QS results in ΔrpfF biofilm overexpression
在盐碱胁迫rpfF基因敲除株(SrM)组中,DSF-QS的缺失促进了敲除株中鞭毛组装、细菌趋化性、转录调控和DNA修复重组相关基因的表达(图3)。其中,鞭毛合成与趋化性运动是生物被膜初始形成的先决条件;参与转录调控的rpoN表达上调最为显著,其编码RNA聚合酶亚基sigma 54,负责调控鞭毛生物合成、生物被膜形成、细菌运动等活动。
In the SrM group, deletion of DSF-QS promoted the expression of genes related to flagellar assembly, bacterial chemotaxis, transcriptional regulation, and DNA repair recombination in ΔrpfF (Fig. 3). Flagellar biosynthesis and chemotactic movement are the prerequisites for the initial formation of biofilm. Gene rpoN, which is involved in transcriptional regulation, is most significantly up-regulated, encoding RNA polymerase sigma 54, responsible for flagellar biosynthesis, biofilm formation, bacterial movement and other activities.
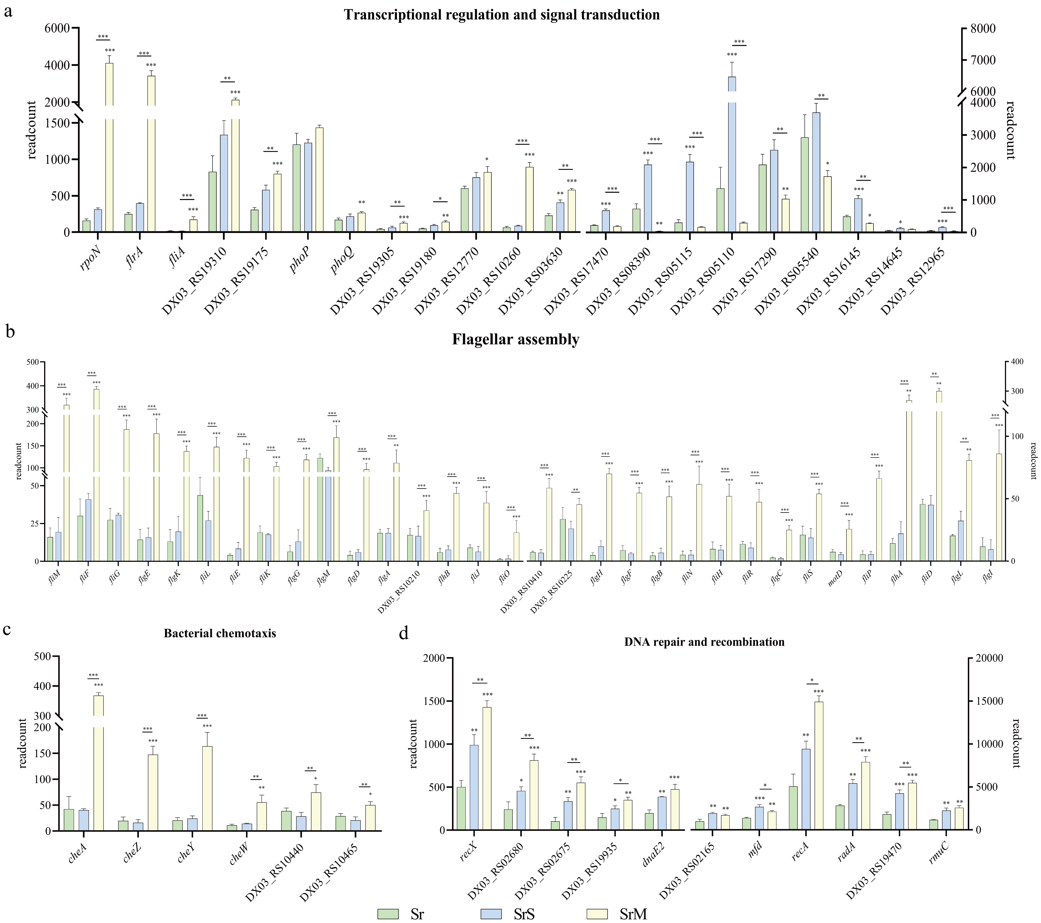
图3 标准培养野生型、盐碱胁迫野生型和盐碱胁迫rpfF基因敲除株代谢活动差异表达基因的比较-2
Fig. 3 Comparison of differential expressed genes (DEGs) in metabolic activities of Sr, SrS and SrM groups-2
通过对生物被膜生物量的测定,发现盐碱胁迫显著抑制了野生型生物被膜形成,而rpfF基因敲除株成膜能力不受盐碱胁迫的影响(图4a);24小时后,rpfF基因敲除株的生物被膜生物量是野生型的4.8倍。同时,rpfF基因敲除株的细胞增殖率低于野生型(图4b, c),但rpfF基因敲除株的成膜速率高于野生型(图4d, e)。
Through the determination of biofilm biomass, it was found that saline-alkaline stress significantly inhibited the biofilm formation of WT, while the biofilm formation ability of ΔrpfF was not affected by saline-alkaline stress (Fig. 4a). After 24 hours, the biofilm biomass of ΔrpfF was 4.8 times that of WT. Furthermore, the cell proliferation rate of ΔrpfF was lower than that of WT (Fig. 4b, c), but the biofilm formation rate of ΔrpfF was higher than that of WT (Fig. 4d, e).
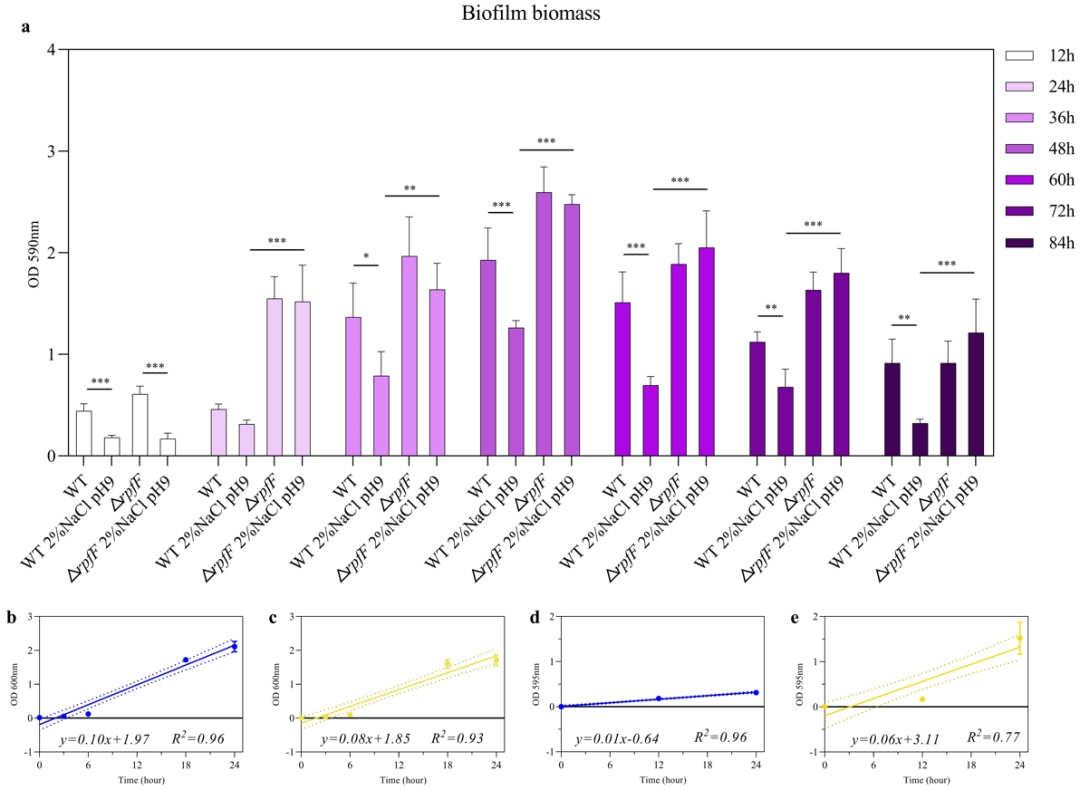
图4 生物被膜定量以及增殖和成膜速率
Fig. 4 Biofilm quantification and rate of proliferation and biofilm formation
(a) Comparison of quantitative analysis of biofilm; (b, c) Growth rate of WT and ΔrpfF during 24 h under saline-alkaline stress; (d, e) Biofilm formation rate of WT and ΔrpfF during 24 h under saline-alkaline stress
三、DSF-QS是嗜根寡养单胞菌根际定殖的关键
DSF-QS is critical for S. rhizophila in rhizosphere colonization
通过qPCR对嗜根寡养单胞菌定殖能力检测发现,具有较强成膜能力的rpfF基因敲除株在根际定殖中不占优势,其在根际土壤中的拷贝数约比野生型低38%(图5)。土壤根际是高度动态的生态系统,因此嗜根寡养单胞菌必须与其他微生物竞争生存资源占据生态位。rpfF基因敲除株无法进行种群增殖、生存代谢与生物被膜形成等生命活动的最优调控(图6),因此在根际竞争中被打败。
The qPCR test showed that ΔrpfF with a stronger biofilm-forming capacity was not dominant in rhizosphere colonization, and its copy number in rhizosphere soil was about 38% lower than that of WT cells (Fig. 5). The rhizosphere is a highly dynamic and complex system composed of microenvironments; therefore, S. rhizophila must compete against other microbes to establish a suitable niche. S. rhizophila ΔrpfF cells were unable to optimally regulate life activities such as population proliferation, survival metabolism and biofilm formation (Fig. 6), so they were defeated in the rhizosphere competition.
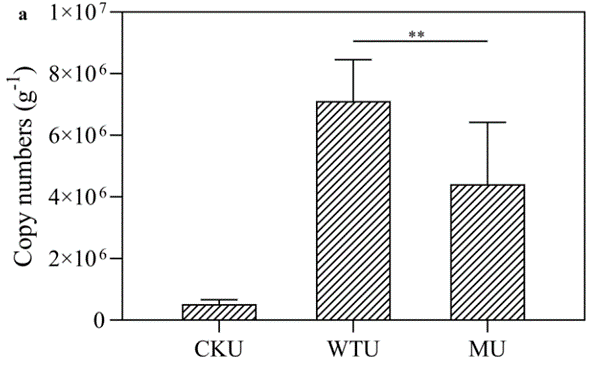
图5 根际土壤中嗜根寡养单胞菌野生型和rpfF基因敲除株拷贝数的比较
CKU、WTU和MU分别表示空白对照、添加野生型和添加rpfF基因敲除株的土壤样品
Fig. 5 Comparison of S. rhizophila WT and ΔrpfF copies in rhizosphere soil
CKU, WTU, and MU indicate the soil samples of control, addition of WT, and addition of ΔrpfF, respectively
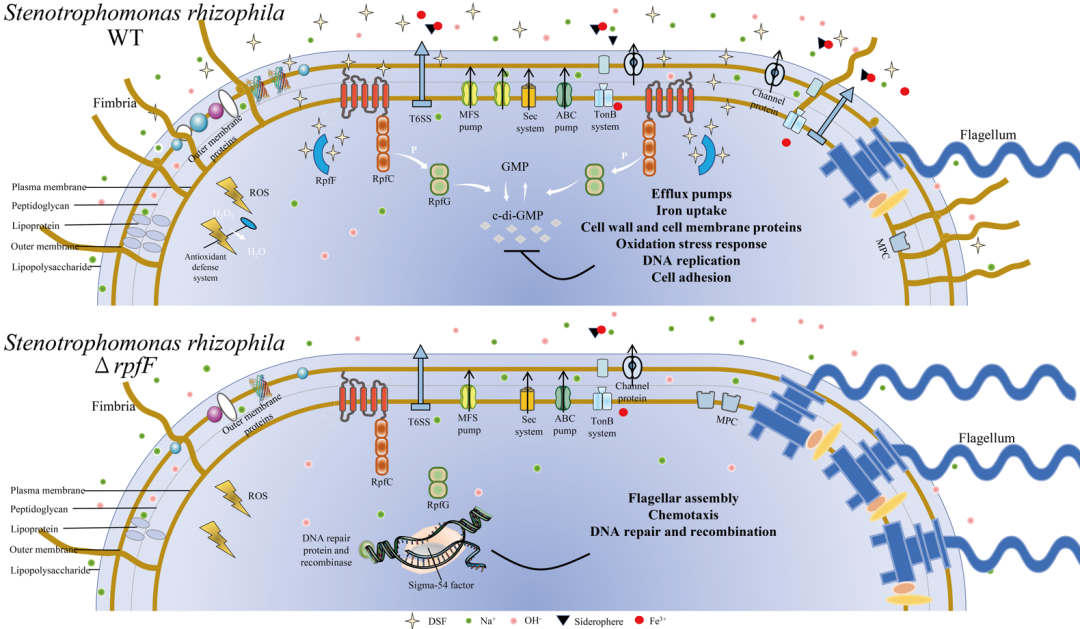
图6 嗜根寡养单胞菌野生型与rpfF基因敲除株应对盐碱胁迫示意图
Fig. 6 Schematic diagram of S. rhizophila WT and ΔrpfF response to saline-alkaline stress
总结 | Conclusions
本研究阐明了DSF-QS调控嗜根寡养单胞菌抵抗盐碱胁迫与种群定殖的机制,在DSF-QS的调控下,野生型增强了细胞的渗透防御、氧化应激反应、细胞黏附、外排泵、抗生物分泌和铁摄取等相关基因的表达,具有较强的耐盐碱胁迫和生存竞争能力,从而确保种群密度。在缺少DSF-QS的情况下,sigma 54和其他转录因子促进鞭毛组装和趋化性运动等高耗能生命活动,过量形成生物被膜,无法保证种群密度,使rpfF基因敲除株在抵抗环境胁迫与定殖中处于劣势。
This study elucidated the mechanism of DSF-QS regulating S. rhizophila resistance to saline-alkaline stress and population colonization. Under DSF-QS regulation, WT cells boosted the expression of genes regulating osmotic defense, oxidative stress response, cell adhesion, efflux pumps, antibiotics biosynthesis, and iron uptake. WT cells have strong saline-alkaline stress tolerance and survival competitiveness, thus ensuring population density. However, in the absence of DSF-QS, sigma 54 and other transcription factors promote flagellar assembly and chemotaxis, highly energy-consuming life activities, excessive biofilm formation. Thus, ΔrpfF failure to ensure population density, which puts ΔrpfF at a disadvantage in resisting environmental stress and colonization.

扫二维码 | 查看原文
https://www.sciencedirect.com/science/article/pii/S0048969722024962?via%3Dihub
本文内容来自ELSEVIER旗舰期刊Sci Total Environ第834卷发表的论文:
Liu Y., Gao J., Wang N., Li X.L., Fang N., Zhuang X. L., 2022. Diffusible signal factor enhances the saline-alkaline resistance and rhizosphere colonization of Stenotrophomonas rhizophila by coordinating optimal metabolism. Sci Total Environ 834, 155403.
DOI: https://doi.org/10.1016/j.scitotenv.2022.155403

通讯作者:庄绪亮 研究员
中国科学院生态环境研究中心
中国科学院青藏高原研究所
中国科学院生态环境研究中心与中国科学院青藏高原研究所研究员、博士生导师。主要从事污染物的微生物转化与调控,流域水、土元素循环微生物生态过程等方面的研究。主持了多项国家重大科技专项以及国家自然科学基金项目等,在有重要影响力的环境科学国际学术期刊发表论文114篇,申请或授权国家发明专利69项。
近2年发表在Sci Total Environ上的论文:
1. Wu et al, 2022. Protistan consumers and phototrophs are more sensitive than bacteria and fungi to pyrene exposure in soil. Sci Total Environ, 822, 153539.
2. Zhou et al, 2021. Enhancing nitrogen removal from anaerobically-digested swine wastewater through integration of Myriophyllum aquaticum and free nitrous acid-based technology in a constructed wetland. Sci Total Environ, 779, 146441.

第一作者:刘颖 博士
中国科学院生态环境研究中心与中国科学技术大学联合培养博士生
中国科学院生态环境研究中心与中国科学技术大学联合培养博士生。主要研究方向为植物—微生物互作、微生物群体感应。以第一作者或共同作者在Science of the Total Environment、Microorganisms、Chemosphere、Environmental Pollution等国际期刊发表论文8篇。
猜你喜欢
10000+:菌群分析 宝宝与猫狗 梅毒狂想曲 提DNA发Nature Cell专刊 肠道指挥大脑
文献阅读 热心肠 SemanticScholar Geenmedical
16S功能预测 PICRUSt FAPROTAX Bugbase Tax4Fun
生物科普: 肠道细菌 人体上的生命 生命大跃进 细胞暗战 人体奥秘
写在后面
为鼓励读者交流、快速解决科研困难,我们建立了“宏基因组”专业讨论群,目前己有国内外5000+ 一线科研人员加入。参与讨论,获得专业解答,欢迎分享此文至朋友圈,并扫码加主编好友带你入群,务必备注“姓名-单位-研究方向-职称/年级”。PI请明示身份,另有海内外微生物相关PI群供大佬合作交流。技术问题寻求帮助,首先阅读《如何优雅的提问》学习解决问题思路,仍未解决群内讨论,问题不私聊,帮助同行。

学习16S扩增子、宏基因组科研思路和分析实战,关注“宏基因组”
点击阅读原文,跳转最新文章目录阅读