A mature laboratory information management system , a network management system integrating pre-processing, inspection, reporting, quality control, statistical analysis, two cancer and other modules . Its development and application will accelerate the process of unification, networking, and standardization of laboratory management.
LIS connects various analytical instruments used in inspection, quarantine, radioimmunoassay, bacteria and microorganisms and scientific research through computer networking to achieve real-time automatic reception, automatic control and comprehensive analysis of data results from various instruments;
The system can be used with barcoding equipment to automatically generate barcodes to reduce errors caused by human factors in laboratory information transmission;
The system provides a simple and fast maintenance method to facilitate users to maintain and manage the entire laboratory department/laboratory or information during use, comprehensively improve the work efficiency of the laboratory department/laboratory, and provide a basis for improving medical quality and clinical diagnosis. Strong guarantee. The LIS system has a built-in standard data interface, which can be fully linked with the hospital information system (HIS) and become a HIS subsystem to realize the sharing of inspection information in the whole hospital.
Technical architecture : ASP.NET CORE 3.1 MVC + SQLserver + Redis

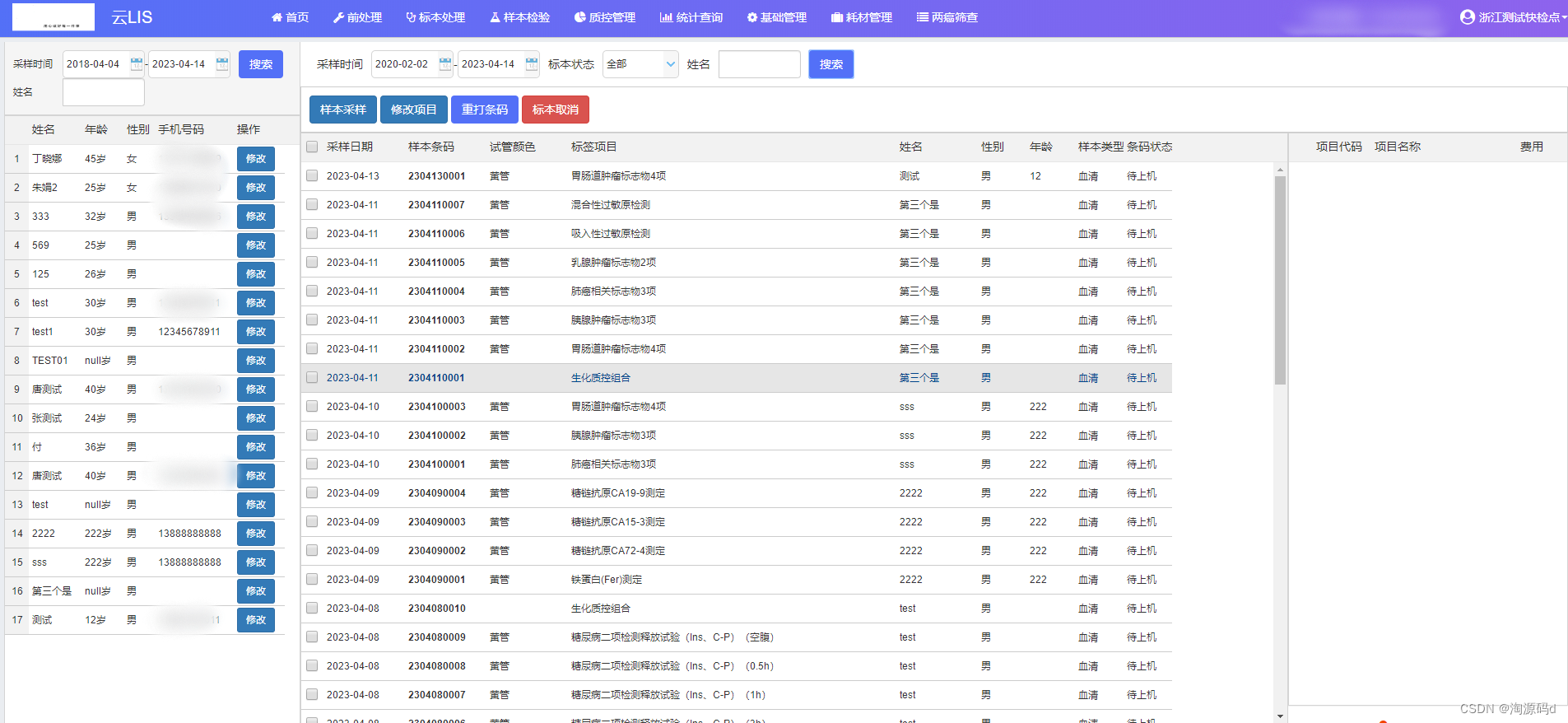
functional module
Sampling module: The sampling technician collects relevant samples for patients and generates corresponding specimen barcode numbers to serve the inspection workstation.
1) Supports both preset barcodes and on-site barcode printing.
2) One or more barcodes can be automatically generated based on classification rules such as test tube category and specimen type.
3) The barcode can be revoked after it is generated.
4) The information on the barcode and receipt slip supports user customization.
Inspection module: Complete inspection process management functions, including specimen acceptance, specimen redoing, result filling, report review, etc., as well as analysis and statistics of various inspection data.
1) Supports one-way communication, LIS automatically receives instrument inspection results.
2) Supporting two-way communication, LIS not only automatically receives instrument inspection results, but also downloads inspection tasks to the instrument.
3) Supports manual entry and modification of inspection results, including single and batch methods, and writes modification records to the log.
4) Supports automatic generation of calculation items, judgment of high and low status of results, and identification of abnormal status of results.
5) Provide comparison of the patient's previous test results.
6) Supports specimen merging and glucose tolerance merging.
7) Individual samples can be modified and deleted, or samples can be modified and deleted in batches.
8) A variety of report styles are provided, and users can also customize the report style.
9) Individual reports can be reviewed, printed and released, or batch reports can be reviewed, printed and released.
10) Reports can be issued to patients or physicians via the network.
Quality control management: perform quality control on the instrument according to the pre-defined quality control rules, and judge whether the instrument is out of control according to the quality control results.
1) Support a variety of quality control rules, such as LJ, WestGard, etc.
2) Supports automatic reception of quality control result data.
3) Support manual entry of quality control data.
4) Realize the intelligent judgment of the out-of-control and in-control status of the instrument.
5) Provide the function of drawing quality control charts, perform quality control analysis, mark out-of-control points, and print out.
Inspection Management
Support the electronic inspection application form in HIS, and the inspection department enters the inspection application form;
Support intelligent determination of sample type according to the type of instrument entered;
The test results are automatically checked for the reference value range, and are prompted with colors;
For test results that exceed normal or critical values, the system will use different signs (sound or text) to remind;
The system supports different rule constraints for the same report operator and reviewer.
Report printing
Supports batch printing of approved inspection reports;
Combined printing of inspection results;
It supports automatic decomposing of the same application form into multiple report forms for printing.
Statistical Analysis
The inspection workload can be counted by time period, inspection equipment or working group;
The inspection workload can be counted according to time period, department, doctor, operator and patient type;
Test results can be classified and counted according to time period, diagnosis, ward and test item name;
Statistical analysis of abnormal results can be performed on the proportion of abnormal results of a certain project according to time period;