Table of contents
- 1. Improved method for quantitative cardiovascular magnetic resonance imaging (CMR)
- 2. Magnetic resonance multitasking
- 3. MRI multi-tasking imaging framework
- 4. Image model and sampling and reconstruction strategy for MRI multitasking
- 5. Rapid 3D steady-state CEST (ss-CEST) imaging using MR multitasking
1. Improved method for quantitative cardiovascular magnetic resonance imaging (CMR)
Physiological movement and other dynamic processes can be conceptualized into multiple temporal dimensions , throughLow-rank tensor (LRT) imaging addresses motion artifacts, enabling motion-resolved quantitative imaging in up to four temporal dimensions. this kindcontinuous acquisitionThe method is called CMR multitasking . Using motion capture rather than motion avoidance does not require the use of ECG triggers or patient breath holding for efficient CMR quantitative imaging.
CMR is imaged during a variety of overlapping dynamics , including physiological (eg, heart beating, respiratory motion) and physical (eg, T1, T2 relaxation) dynamics, complicating the imaging process. Traditional strategies for dealing with dynamic overlap in cardiovascular imaging have been to "freeze" as much dynamic as possible using complex electrocardiogram (ECG) controls, patient breath-holds, and/or brief acquisitions , but this means thatGive up useful time for the rest of the dynamic,andEach dynamic in the acquisition requires a combination of freezing mechanisms corresponding to different dynamics。
For unhealthy subjects with cardiac arrhythmias or difficulty holding breath, CMR examinations are lengthy.
2. Magnetic resonance multitasking
MRI multitasking is a continuous acquisition framework that canSimultaneously resolve the many overlapping dynamics involved in quantitative cardio-cerebrovascular imaging. Magnetic resonance multitasking conceptualizes different sources of image dynamics into different temporal dimensions,Leverage multitasking to capture (rather than avoid) motion, slack, and other dynamics, for time-resolved T1 mapping that corrects the signal saturation problem in dynamic contrast-enhanced (DCE) imaging .
The LRT image model allows MR multitasking, exploits the correlation between images at different time points to reduce sampling requirements (also reduces sampling through compression, MR fingerprinting), breaks the "exponential increase in scan time with dimensionality" growth" curse of dimensionality . The degrees of freedom of LRT and its required scan time are approximately linear with the number of dimensions, making it suitable for multidimensional imaging.
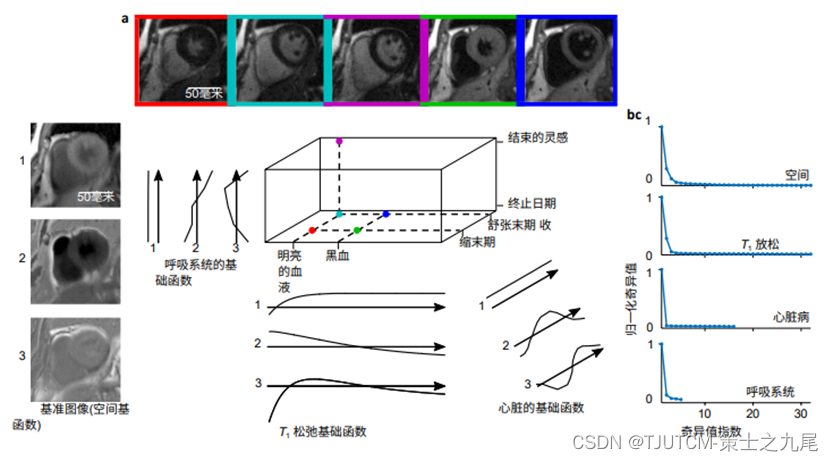
a. Locations of different images in three temporal dimensions. different T, the weighting is on the inverted time axis (horizontal) ,Different cardiac phases are located on the cardiac time axis (depth),The different breathing periods are located on the breathing time axis (vertical)
b. The three most important basis functions describing each dimension of the image tensor , reconstructed from 1 minute of data
c. Raw subspace training data from the singular value curve of the higher order svD at 12.3 minutes ( i.e. enough data to covering all motion state and contrast combinations ), showing that the singular values decay rapidly for all unrolling of the original data tensor
Magnetic resonance multitasking addresses many longstanding limitations in quantitative CMR :
(1)efficiently handles movement, which eliminates the reliance on ECG control and/or breath-hold , providing a potential means for performing quantitative CMR in patients with arrhythmias.
(2) Corrected inaccuracies in quantifying contrast agent concentrations from T1-weighted imaging, allowing quantification from single contrast agents .
(3) simplified workflow, using a single setup-free scan to generate co-registered(?), motion-resolved parametric maps ,rather than a series of dislocation scans, each scan involves a complex setup process to determine ECG trigger delay time, respiratory gating window center and bandwidth, and/or select timing parameters for appropriate image contrast.
This method allows flexible sampling and efficient factor tensor reconstruction . Unlike MR fingerprinting, in addition to NMR relaxation, it alsoImaging motion and DCE. Furthermore, since CMR multitasking with LRT imaging can scan a wide range of "natural" imaging contrasts (i.e., as opposed to the random image contrast of MR fingerprinting), the images produced by multitasking may also in turn validate their accuracy.
LRT imaging is different from other multi-dimensional imaging methods , such as XD-GRASP, which exploits the "local" similarity of images by implicitly assuming a constant evolution of transverse slices along each time dimension, while LRT imaging comprehensively exploits the There is correlation both horizontally and diagonally of the image in space .
3. MRI multi-tasking imaging framework
CMR multitasking represents a set of cardiovascular and cerebrovascular images as a multi-dimensional tensor (or array) , where one dimension indexes the voxel position (ie, combined with the spatial dimension), and the other dimensions index N different time dimensions ,Each dimension corresponds to a different "task" to be imaged. By low-rank modeling this tensor, weDescribe and exploit the relevance of images(along the diagonals of each time dimension and the entire multidimensional time dimension).
A memory- and time-efficient factorial approach is developed for image reconstruction by interleaving sparsely sampled image data with training data from an auxiliary subspace that frequently samples a subset of K-space, where the core tensor and N time The factor matrix is estimated from the subspace training data, and the spatial factor matrix is recovered by fitting the core tensor and temporal factor matrix to the rest of the measurement data.
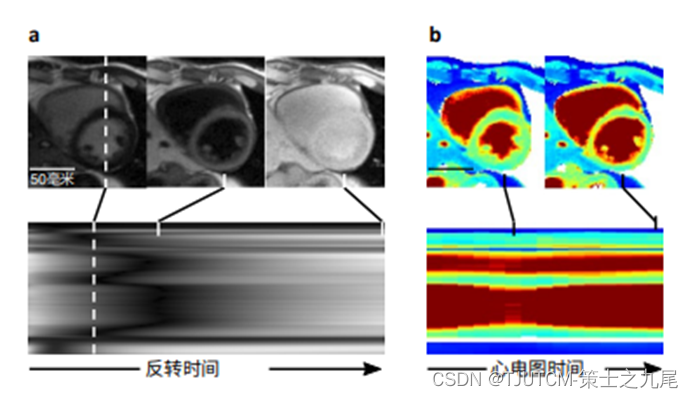
a. CMR multitasking produces finely resolved contrast changes along the inverted time dimension
b. Cardiac resolved T1 maps produced by multitasking
4. Image model and sampling and reconstruction strategy for MRI multitasking
CMR multitasking represents cardiovascular images as a multidimensional function I(x,t1,t2,...tN) of spatial position and N time dimensions t1,t2,...tN .Each time dimension corresponds to a different "task" to be solved, e.g. the time dimension corresponds to cardiac motion, respiratory motion, time after magnetization preparation (signal evolution along the way depends on tissue property parameters such as T1 and T2) and time after imaging initiation (useful for describing time through).
Although the LRT model releases the sampling requirement from the curse of dimensionality , theThe size of N+1 way tensor (or multidimensional array) A is still growing exponentially. Thus, the image I is stored in uncompressed form by an auxiliary variable of the same size involved in the Singular Value Decomposition (SVD) thresholding solution as an N+1-way tensor (or multidimensional array) A pair of memory The requirements are very high. Furthermore, each iteration of the image reconstruction algorithm may involve operations on each column of A (which may number in the hundreds of thousands) , as well as multiple SVDs of large matrices, all of which require significant computation time.
The training data needs to be sampled more frequently than other locations in k-space. This subset of data ("subspace training data") contains limited spatial information but a large amount of temporal information suitable for determining Φ. Data is collected frequently enough to address the finest physiological questions.
The sampling performance of ordered uniform sampling is enhanced by changes in cardiac and respiratory rates if ordered uniform sampling is consistent with a time-based function, such as periodic sampling synchronized with the respiratory cycle, cardiac cycle, or magnetization preparation period. The magnetization preparation schedule is usually just periodic, but the preparation and sampling periods are chosen to avoid mutual synchronization. To avoid these problems, sampling schemes such as golden-angle radial sampling or random Cartesian sampling can be used, which provide incoherence even in the presence of periodic motion.
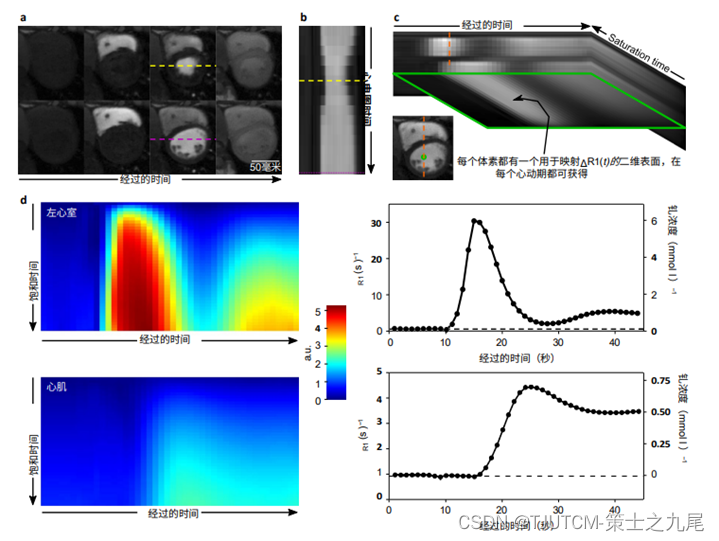
a. The systolic and diastolic cardiac phases in which contrast dynamics are captured
b. This is due to the method's ability to resolve cardiac motion
c. The elapsed time dimension (used to depict contrast dynamics) and the saturation time dimension combine to yield two dimensional signal intensity surfaces instead of the conventional 1D signal intensity curves
d. These signal intensity surfaces are used to plot R(t), which takes signal saturation into account and directly yields Gd concentration after linear transformation
5. Rapid 3D steady-state CEST (ss-CEST) imaging using MR multitasking
5.1 Introduction to fast three-dimensional steady-state CEST (ss-CEST) imaging using MR multitasking
Chemical exchange saturation transfer is a non-contrastive MRI technique that indirectly detects exchangeable elements in a pool by. Chemical exchange saturation transfer MRI provides a new contrast mechanism to image important physiological information such as pH and me-tabolite concentration between the exchanged proton pool and the water pool.Images are collected at different saturation frequencies, resulting in the so-called Z-spectrum, which reflects a steady-state signal shifted by the sampling frequency at a given saturation power.
The broad and symmetric coverage of the Z-spectrum allows multi-cell analysis while revealing different CEST effects (e.g., amide proton transfer (APT), relay nuclear Overhauser enhancement (rNOE) effects), as well as other application-specific effects (e.g., glycated CEST, CrCEST and glycated NOE). For reliable multi-cell analysis, dense sampling of broad Z spectra is often required. Considering that tens of frequency offsets are typically sampled, the acquisition time per frequency offset (including long saturation blocks) is best limited to a few seconds to keep scan times acceptable in clinical practice. This time constraint usually only allows a single k-space acquisition for each frequency offset after the saturation module. This single-shot scheme makes fast, high-quality 3D CEST imaging difficult .
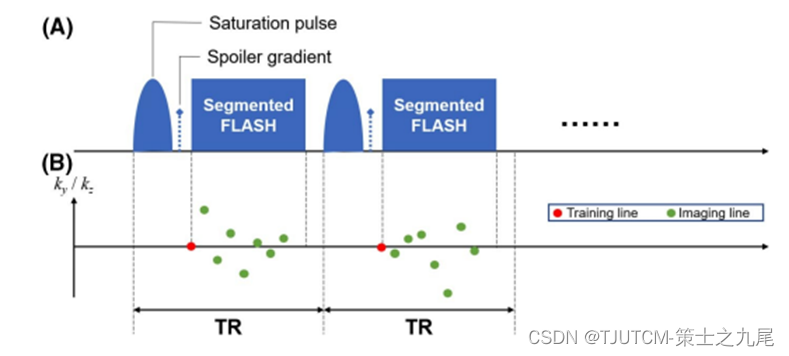
(A) Sequence design: each module (TR=70 ms) contains a single-leaf Gaussian saturation pulse (txat=30 ms, flip angle=500°), followed by a spoiler gradient and eight FLASH readout lines (one training line plus seven imaging lines; flip angle = 5°)
This module is repeated at a specific frequency offset (Nw = 80) and then switched to another
(B) k-space sampling mode: in each module , first the "training line" (central k-space line) is obtained, then seven "imaging lines" (pseudo-randomly sampled lines with Gaussian distribution in the xy and ga directions)
5.2 Rapid 3D steady-state CEST (ss-CEST) imaging using MR multitasking
Scan times are reduced by optimizing k-space sampling efficiency , such as snapshot gradient echo (GRE) readout with helical center reordered k-space acquisition, or 3D EPI readout with CAIPIRINHA downsampling. It can provide a 2-plane resolution of 1.7 x 1.7 mm with a FOV of 220 x 180 x 54 mm, acquired in 7 seconds per offset using 3D GRE readout, and 4.3 seconds per offset using 3D EPI readout 1.8 mm isotropic resolution with FOV of 256 × 224 × 156 mm
A potentially faster method, the Steady-State CEST (ss-CEST) method , pre-saturates and k-space samples in an interleaved pattern of repeating modules to Ensuring that the saturated exchange steady state remains constant for most of the time at each frequency offset, the interleaved mode provides more flexibility in sequence design and possible speedup.
But the original ss-CEST method needs more than 12 minutes to acquire the whole Z-spectrum, which is still too long for practical use. We can combine radial readout with multi-line singular value decomposition to further reduce the total scan time to less than 5 minutes. Compared with the previous ss-CEST method, the acquisition time per frequency offset was reduced from more than 10 seconds to 7.6 seconds.
Magnetic resonance multitasking as a low-rank tensor imaging strategy. Correlations between images acquired at different frequency offsets, as well as data during near-steady state periods, are exploited by low-rank tensor modeling to reduce scan time and improve image quality. This allowed a spatial resolution of a Z-spectrum covering the whole brain of 1.7 × 1.7 × 3.0 mm to be obtained within 5.5 minutes.
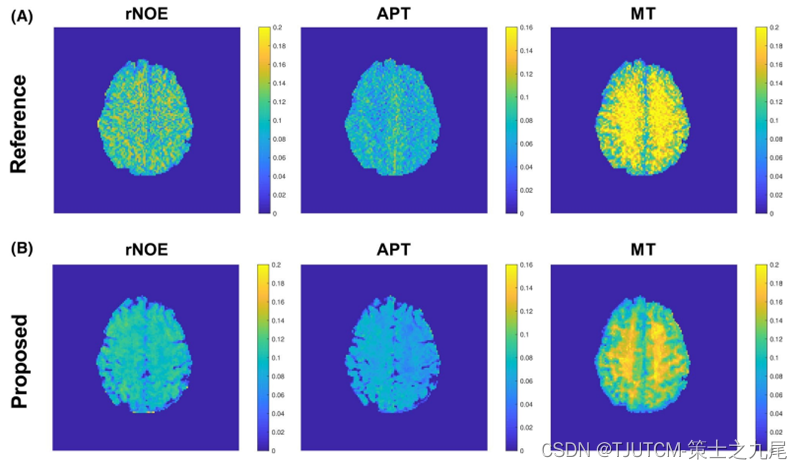
Note that the slice thickness used in the 2D single-shot FLASH CEST method is 10 mm, which is larger than 3 mm in the proposed 3D multitasking ss-CEST method. Therefore, although the slice centers match between (A) and (B), the spatial coverage is not exactly the same.
5.3 Discussion on fast three-dimensional steady-state CEST (ss-CEST) imaging using MR multitasking
Compared to single-shot acquisition methods (or pseudo-steady-state methods) , the ss-CEST method ensures that the steady state of the chemical exchange process is maintained for most of the acquisition time. It allows continuous acquisition for more efficient traversal of K-space compared to single-shot methods. But in the original ss-cEST implementation, only in parallel imaging have additional sources of acceleration
and in MR-Multitasking the low-rank tensor modulus enforces synthesis with respect to two separate sequence parameter dimensions: frequency offset and time after frequency increase The low-rank nature of the spatial dimension (so no assumptions are made about the spatial structure). Additionally, multitasking systems employ this mode during image reconstruction to speed up acquisition.
In addition to speeding up the acquisition, the multitasking ss-CEST method has two additional advantages:
(1) the steady-state method at each frequency offset is modeled, and the signal before the steady-state is excluded ,Allows quantification using uncorrupted steady-state values。
(2)The Z-spectrum is automatically denoised under the low-rank constraint. Considering enough spatio-temporal relationships, the only clear limiting factor for the speedup is the time to reach a steady state at each frequency offset. The multitasking ss-CEST method
can be further optimized . First, we can take advantage of advanced k-space sampling trajectories , such asnon-cartesian trajectory(like a helix), potentially improving sampling efficiency and incoherence over Cartesian acquisition ,Further reduce scan time and improve image quality.
Secondly, you canFurther optimization of sampling modes for specific frequency offsets, to reduce the total number of sampling frequencies, so that the total scan time can be reduced while maintaining the reliability and robustness of the multi-pool fitting data.