Preface
As we all know, the establishment of production facilities in the food, pharmaceutical, and industrial industries looks very similar, but the production environment and requirements between them are completely different. There are a series of legislative requirements and guidelines for the food, pharmaceutical, and chemical industries. Much more onerous.
The EXOR food/drug series industrial control panel provided by Guangzhou Hongke is developed in accordance with the hygienic design standard** (DIN EN1672-2, EHEDG and FDA 21 CFR 177.2006)**, which can perfectly satisfy the hardware appearance, performance and function. Special environmental application requirements in the food/pharmaceutical industry.
Brand hard power
The HMI provided by Hongke has high performance, and its appearance is also processed and configured for the characteristics of the food and beverage & pharmaceutical industries, which has strong adaptability and reliability. details as follows:
The HMI front panel frame is made of stainless steel, and the panel glass is treated with polyester coating, making the front panel have a high protection level of IP69, ensuring that the HMI can withstand frequent washing and washing with high temperature (up to 80°C) and high pressure water, even It is a highly corrosive cleaning agent, and it is also completely "holdable".
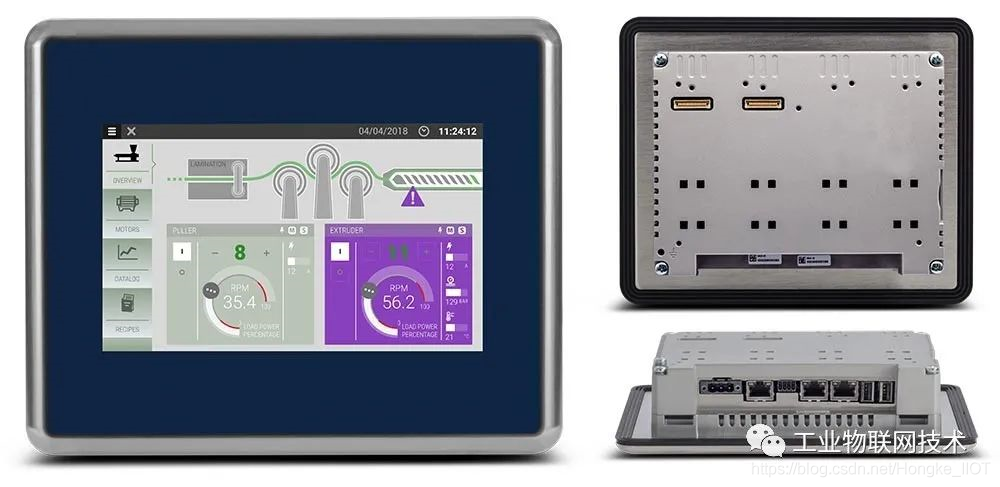
In addition, this HMI provided by Hongke also has an intimate detail design for the food and pharmaceutical industry: the four corners of the stainless steel front panel frame are highly polished, which makes the frame and the machine surface have the best contact angle. It also makes the HMI easier to clean, and the cleaning fluid can flow away more easily during the flushing process, and no bacteria or microorganisms remain.
In this way, this HMI of Hongke can eliminate the accumulation of food, oil, product powder particles, food, pharmaceutical and chemical industries no longer need to configure expensive cabinets for HMI equipment, which not only eliminates the worries of this type of industry, but also Greatly save the cost of equipment.
Brand soft power
To ensure information security, the HMI products provided by Hongke meet the requirements specified in FDA 21 CFR Part 11, and provide a complete and reliable electronic signature, electronic record and audit trail security solution for the safe processing of industrial applications. The details are as follows:
(1) Electronic signature The
x.509 certificate can be used to sign the report generated by the HMI product configuration software JMobile Studio provided by Hongke.
(2) System security access
Only when the user enters the account password, the user can log in to the system. It can also be configured to force the user to log in to the system with the account password when the user performs remote access through the HMI Client; the
user can change his password at any time, but the administrator It can be set after a period of time that users must change the password or redefine each user’s password and force them to redefine it when they log in for the first time; it
can be set that after users log in to the system, they need to enter again before making any changes to the system/project Password confirmation; the
system will ensure that two users with the same ID cannot be defined.
(3) User rights management
application developers assign different levels of users (administrators or operators) to different levels of users (administrators or operators) according to actual needs, which can access and operate different rights on the HMI device, including: all controls that can be used in the project /Actions, etc. Define the control/action access permissions at the project level, page level, or control level.
(4) Audit trail
All operations performed by the user on the selected resource will be automatically recorded in the operation log. The recorded content includes: timestamp, user name that performed the operation, and information related to the modified resource; the
operator cannot modify it Logs, log reports can be exported as .csv or .pdf files;
if an unauthorized user attempts to operate the system, the failed operation will be recorded in the log, and the system security unit and organization can be checked from the administrator Management report.
(Finish)